'A' Level Atomic Structure Questions
Q1. A neutral atom of carbon is represented by 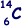 .
.
(i) Name the constituents of this atom and state how many of each are present.
(ii) Which constituent of an atom has the largest charge-to-mass ratio?
(iii) Carbon has several isotopes. Explain the term isotope.
(Total 6 marks)

Q2.
(a)  is a neutral atom of thorium. How many protons, neutrons and electrons does it contain?
is a neutral atom of thorium. How many protons, neutrons and electrons does it contain?
(2)
(b)  is a neutral atom of a different isotope of thorium which contains Z electrons. Give possible values for X, Y and Z.
is a neutral atom of a different isotope of thorium which contains Z electrons. Give possible values for X, Y and Z.
(3)
(Total 5 marks)

Q3.
(a) The most abundant isotope of cobalt is represented by 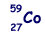 How many protons, neutrons and orbital electrons are there in a neutral atom of this element?
How many protons, neutrons and orbital electrons are there in a neutral atom of this element?
(2)
(b) How is the nuclide that has one less proton than the nickel nuclide,  , represented?
, represented?
(2)
(c)
(i) The heaviest isotope of hydrogen, whose nucleon number is 3, is called tritium. How is tritium represented?
(ii) Calculate the charge per unit mass, in C kg–1, for a tritium nucleus.
(3)
(Total 7 marks)

Q4.
An atom of argon 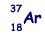 is ionised by the removal of two orbiting electrons.
is ionised by the removal of two orbiting electrons.
(a) How many protons and neutrons are there in this ion?
(2)
(b) What is the charge, in C, of this ion?
(2)
(c) Which constituent particle of this ion has:
(i) a zero charge per unit mass ratio,
(ii) the largest charge per unit mass ratio?
(2)
(d) Calculate the percentage of the total mass of this ion that is accounted for by the mass of its electrons.
(3)
(Total 9 marks)

Q5. A radioactive isotope of carbon is represented by 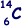
(a) Using the same notation, give the isotope of carbon that has two fewer neutrons.
(1)
(b) Calculate the charge on the ion formed when two electrons are removed from an atom of 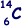 .
.
(2)
(c) Calculate the value the charge to mass ratio for the nucleus of an atom of 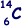 .
.
(2)
(Total 5 marks)

Q6.
(a) How many protons, neutrons and electrons are there in an atom of 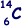 ?
?
(2)
(b) The atom loses two electrons.
For the ion formed;
(i) calculate its charge in C,
(ii) state the number of nucleons it contains,
(iii) calculate the charge to mass ratio in C kg–1.
(4)
(Total 6 marks)

Q7.
(a) An ion of plutonium  has an overall charge of +1.6 × 10–19C.
has an overall charge of +1.6 × 10–19C.
For this ion state the number of
(i) protons (ii) neutrons (iii) electrons
(3)
(b) Plutonium has several isotopes.
Explain the meaning of the word isotopes.
(2)
(Total 5 marks)

Q8.
(a) Name the constituent of an atom which
(i) has zero charge,
(ii) has the largest charge to mass ratio,
(iii) when removed leaves a different isotope of the element.
(3)
(b) An  particle is the same as a nucleus of helium,
particle is the same as a nucleus of helium,  .
.
The equation





represents the decay of thorium by the emission of an  particle.
Determine:
particle.
Determine:
(i) the values of X and Y, shown in the equation,
(ii) the ratio of the mass of the radium nucleus to the mass of the alpha particle
(3)
(Total 6 marks)

Q9.
(a) A stable atom contains 28 nucleons.
Write down a possible number of protons, neutrons and electrons contained in the atom.
(2)
(b) An unstable isotope of uranium may split into a caesium nucleus, a rubidium nucleus and four neutrons in the following process.
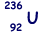

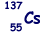

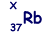

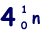
(i) Explain what is meant by isotopes.
(ii) How many neutrons are there in the 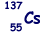 nucleus?
nucleus?
(iii) Calculate the charge to mass ratio, in C kg–1, for the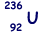 nucleus.
nucleus.
(iv) Determine the value of X for the rubidium nucleus.
(6)
(Total 8 marks)

Q10.
(a) Give the number of nucleons and the number of electrons in an atom of Na .
.
(2)
(b) The isotope  is a positron emitter. In positron emission an up quark undergoes the following change:
is a positron emitter. In positron emission an up quark undergoes the following change:
u  d + e+ + ve
d + e+ + ve
Show that charge, lepton number and baryon number are conserved in this decay.
(3)
(c) Describe what happens when a positron collides with an electron.
(2)
(Total 7 marks)

Q11.
(a)
(i) Determine the charge, in C, of a  nucleus.
nucleus.
(ii) A positive ion with a  nucleus has a charge of 4.80 × 10–19 C. Determine how many electrons are in this ion.
nucleus has a charge of 4.80 × 10–19 C. Determine how many electrons are in this ion.
(4)
(b) A  nucleus may decay by emitting two
nucleus may decay by emitting two  – particles to form a plutonium
– particles to form a plutonium  nucleus . State what X and Y represent and give the numerical value of each.
nucleus . State what X and Y represent and give the numerical value of each.
(4)
(Total 8 marks)

Q12.
(a) State what is meant by the specific charge of a nucleus and give an appropriate unit for this quantity.
(2 marks)
(b) Nucleus X has the same nucleon number as nucleus Y. The specific charge of X is 1.25 times greater than that of Y.
(i) Explain, in terms of protons and neutrons, why the specific charge of X is greater than that of Y.
(2 marks)
(ii) Nucleus X is  . Deduce the number of protons and the number of neutrons in nucleus Y.
. Deduce the number of protons and the number of neutrons in nucleus Y.
(4 marks)
(Total 8 marks)

Q13.
(a) The table below contains data for four different nuclei, P, Q, R and S.
Nucleus |
Number of neutrons |
Nucleon number |
P |
5 |
11 |
Q |
6 |
11 |
R |
8 |
14 |
S |
9 |
17 |
(i) Which nucleus contains the fewest protons?
(1 mark)
(ii) Which two nuclei are isotopes of the same element?
(1 mark)
(iii) State and explain which nucleus has the smallest specific charge.
(2 marks)
(Total 4 marks)

Q14.
(a) The nucleus of a particular atom has a nucleon number of 14 and a proton number of 6.
(i) State what is meant by nucleon number and proton number.
(1 mark)
(ii) Calculate the number of neutrons in the nucleus of this atom.
(1 mark)
(iii) Calculate the specific charge of the nucleus, in C kg-1.
(3 marks)
(b) The specific charge of the nucleus of another isotope of the element is 4.8 × 107 C kg–1.
(i) State what is meant by an isotope.
(2 marks)
(ii) Calculate the number of neutrons in this isotope.
(3 marks)
(Total 10 marks)

Q15. The table belowcontains five statements that refer to isotopes and some radium isotopes.
|
 |
 |
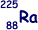 |
 |
Isotope with the smallest mass number
|
 |
|
|
|
Isotope with most neutrons in nucleus |
|
|
|
|
Isotope with nucleus which has the largest specific
charge |
|
|
|
|
Isotope decays by β– decay to form 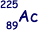 |
|
|
|
|
Isotope decays by alpha decay to form 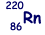 |
|
|
|
|
(a) Complete the table by ticking one box in each row to identify the appropriate isotope for each statement. The first row has been completed for you.
[4 marks]
(b)
(i) An atom of one of the radium isotopes in the table is ionised so that it has a charge of +3.2 × 10–19 C. State what happens in the process of ionising this radium atom.
[1 mark]
(ii) The specific charge of the ion formed is 8.57 × 105 C kg–1. Deduce which isotope in the table has been ionised. Assume that both the mass of a proton and the mass of a neutron in the nucleus is 1.66 × 10–27 kg.
[3 marks]
(Total 8 marks)







