Solutions: Atomic Structure
Q4.
An atom of argon 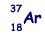 is ionised by the removal of two orbiting electrons.
is ionised by the removal of two orbiting electrons.
(a) How many protons and neutrons are there in this ion?
18 protons 
(37 - 18 gives) 19 neutrons 
(2)
(b) What is the charge, in C, of this ion?
Two electrons have been removed so the charge is positive You are asked for the charge in coulomb so saying +2 won't get you the mark! Q = 2 x 1.6 x 10-19 C = 3.2 x 10-19 C
You are asked for the charge in coulomb so saying +2 won't get you the mark! Q = 2 x 1.6 x 10-19 C = 3.2 x 10-19 C 
(2)
(c) Which constituent particle of this ion has:
(i) a zero charge per unit mass ratio,
neutron 
(ii) the largest charge per unit mass ratio?
electron 
(2)
(d) Calculate the percentage of the total mass of this ion that is accounted for by the mass of its electrons.
ratio = (16 x 9.11 x 10-31 )/(37 x 1.67 x 10-27
)/(37 x 1.67 x 10-27 ) = 2.36 x 10-4 percentage = 2.36 x 10-2 %
) = 2.36 x 10-4 percentage = 2.36 x 10-2 % 
(3)
(Total 9 marks)


