(ii) Calculate the number of neutrons in this isotope.
Let
N = the nucleon number and
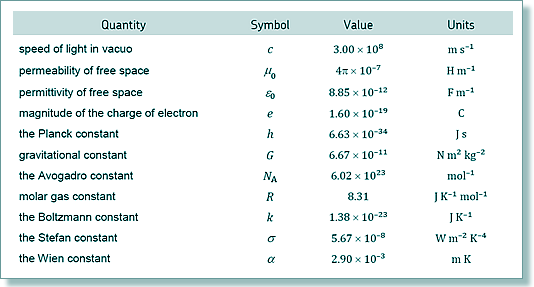
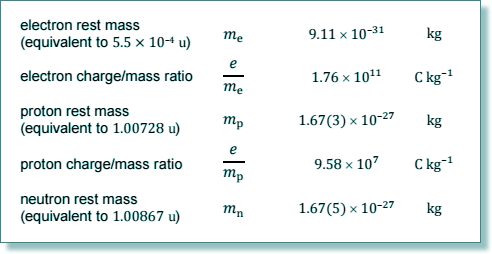
the mass of a nucleon be aproximated to the average of 1.674 x 10-27
Then:
4.8 x 107 = 6 x 1.60 x 10-19/(N x 1.67 x 10-27)
N = 6 x 1.60 x 10-19/(1.67 x 10-27x 4.8 x 107) 
------------------------------------------------------
OR reasoned another way....
Both of these have the same number of protons, but different numbers of neutrons.
specific charge A = QA/mA
specific charge B = QB/mB
specific charge A /specific charge B = QAmB/QBmA
But QA = QB
so, specific charge A /specific charge B = mB/mA
(4.10 x 107)/(4.8 × 107) = mB/mA
4.1 /4.8 = mB/mA
As the mass of the neutron and proton are virtually the same....
mB/mA = nucleon number of B/nucleon number of A
mB/mA = 0.854 
so, nucleon number of B = 0.854
x 14 = 12
-----------------------------------------------------------
Therefore the nucleon number of A = 12
And the number of neutrons = 12 - 6 = 6