Solutions: Atomic Structure
Q1. A neutral atom of carbon is represented by 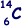 .
.
(i) Name the constituents of this atom and state how many of each are present.
- protons and neutrons

- 6p , 8n

- [or u and d quarks ,
 20u and 22d
20u and 22d  ]
]
- 6 electrons

(ii) Which constituent of an atom has the largest charge-to-mass ratio?
- electron

(iii) Carbon has several isotopes. Explain the term isotope.
Isotopes are nuclei that contain identical numbers of protons but different numbers of neutrons 
(Total 6 marks)


