Solutions: Atomic Structure
Q9.
(a) A stable atom contains 28 nucleons.
Write down a possible number of protons, neutrons and electrons contained in the atom.
number of protons = number of electrons (e.g.14) 
number of protons + number of neutrons = 28 
(2)
(b) An unstable isotope of uranium may split into a caesium nucleus, a rubidium nucleus and four neutrons in the following process.
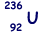

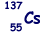

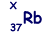

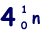
(i) Explain what is meant by isotopes.
Isotopes are nuclei with nuclei with the same number of protons  but different number of neutrons/nucleons
but different number of neutrons/nucleons 
(ii) How many neutrons are there in the 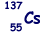 nucleus?
nucleus?
137 – 55 = 82 
(iii) Calculate the charge to mass ratio, in C kg–1, for the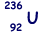 nucleus.
nucleus.
Q/m = (92 x 1.6 x 10-19)/(236 x 1.67 x 10-27)  = 3.73 × 107 C kg–1
= 3.73 × 107 C kg–1 
(iv) Determine the value of X for the rubidium nucleus.
X = 236 – 137 – 4 = 95 
(6)
(Total 8 marks)


