Solutions: Atomic Structure
Q15. The table belowcontains five statements that refer to isotopes and some radium isotopes.
(a) Complete the table by ticking one box in each row to identify the appropriate isotope for each statement. The first row has been completed for you.
[4 marks]
(b)
(i) An atom of one of the radium isotopes in the table is ionised so that it has a charge of +3.2 × 10–19 C. State what happens in the process of ionising this radium atom.
It has lost two electrons 
[1 mark]
(ii) The specific charge of the ion formed is 8.57 × 105 C kg–1. Deduce which isotope in the table has been ionised. Assume that both the mass of a proton and the mass of a neutron in the nucleus is 1.66 × 10–27 kg.
The charge on the ion is given you in part (i).
Specific charge = charge/mass
mass = charge/specific charge = 3.2 × 10–19/8.57 × 105 = 3.73 x 10-25
mass = number of nucleons x 1.66 × 10–27
number of nucleons = 3.73 x 10-25/1.66 × 10–27 = 225 
The isotope is 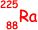


[3 marks]
(Total 8 marks)


