Solutions: Atomic Structure
Q6.
(a) How many protons, neutrons and electrons are there in an atom of 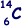 ?
?
6 (protons) and 6 (electrons) 
8 (neutrons) 
(2)
(b) The atom loses two electrons.
For the ion formed;
(i) calculate its charge in C,
Q = (2 × 1.6 × 10–19) = 3.2 × 10–19 C 
(ii) state the number of nucleons it contains,
14 
(iii) calculate the charge to mass ratio in C kg–1.
m = 14 × 1.67 × 10–27 kg 
Q/m = (answer to (i))/(14 × 1.67 × 10–27) = 1.4 × 107 C kg–1 (1.37 ×107 C kg–1)
(1.37 ×107 C kg–1)
(allow C.E for values from (i) and (ii))
(4)
(Total 6 marks)


