Half Life and Rate of Decay
Or you could define it as: The time it takes for the count rate (or activity) of a sample containing the isotope to fall to half its initial level - after elimination of background rate.
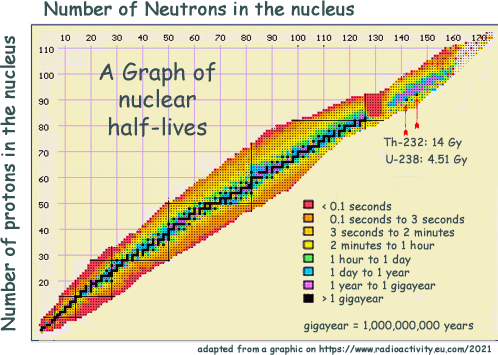
Range of half-lives
This map of nuclei shows the massive range of half-lives that isotopes have - from less than a second to billions of years. The black central line plots nuclei that are either stable or have a half-live longer than a billion years. You can see that the further away anisotope is from that stable line the shorter the half life is. Those nuclei which only survive for a fraction of a second, (coloured in red) are found at the outermost edges of the plot. Those are mainly 'man made' - they would not exist for very long in nature for us to have discovered them! A group of very heavy, very unstable nuclei can also be found around the 130 neutron, 85 proton mark. Among even heavier nuclei, we find an island of comparative stability (in mauve), where a group of isotopes have a half-life between the one- and the billion-year mark. The two heaviest natural nuclei, thorium 232 and uranium 238, are the solitary black dots in the mauve island. Hazard related to Half LifeYou may think that those with very short half lives do not matter - but they do. A sample containing a billion short living isotope nuclei will deliver a very intense burst of radiation instantly - whereas a similar sized sample of long half life nuclei would register very little activity... but would remain radioactive for a long time. When performing tasks you need an isotope with a suitable half life. GCSE standard questions on half lifeAt GCSE you do not have the maths skills to cope with the equations in the A Level section below. You just need to know that the half-life is the time taken for half on the sample you have to decay. Working out a half-life for a GCSE standard question is best explained with an example:
After 14 minutes the count rate had dropped to 5 Bq. What would be the half-life? Well, it would take:
So it would take seven half lives for the activity to drop from 640 Bq to 5 Bq. The time for this to happen was 14 minutes 14 ÷ 7 = 2 so the half-life must be 2 minutes. Sometimes questions include a value for the background rate. This is the rate of activity that is due to background radiation NOT the sample and must therefore be deducted before any calculations are done.
It took 10 minutes for the count-rate to drop from 102 Bq to 12 Bq when a radioactive substance's count rate was being measured. What was the half-life of the sample? Initial count-rate due only to the sample was (102-6) Bq = 96 Bq Final count-rate due
only to the sample was (12-6) Bq = 6 Bq It would take:
So it would take four half lives for the activity of the sample alone to drop from 96 Bq to 6 Bq. The time for this to happen was 10 minutes so the half-life must be 2.5 minutes.
|
Follow me...
|



 Suppose
you have a radioisotope producing a count rate of 640 Bq.
Suppose
you have a radioisotope producing a count rate of 640 Bq.
 Rearranging we get that the half life of a radioactive
isotope is inversley proportional to the decay constant.
Rearranging we get that the half life of a radioactive
isotope is inversley proportional to the decay constant. 


