Experiments with radioactive substances
Note to teachers

 Safety issues are very important when dealing with radioactive isotopes. See here.
Safety issues are very important when dealing with radioactive isotopes. See here.

You may want to use a simulation instead of real sources -
See here.
 It is a useful rule
of thumb to know that the activity of a sample drops to less than 1% of
its value in seven half lives (see Tc99-m)
It is a useful rule
of thumb to know that the activity of a sample drops to less than 1% of
its value in seven half lives (see Tc99-m)
Practical radioactivity investigations
The
activity of a sample can be measured with a Geiger-Müller
tube connected to a rate-meter or by connecting it to a scaler and
timing how long you allow the scaler to count for.

The above photo is of the wonderful antique device we had at my school!
 See here for how a Geiger-counter works
See here for how a Geiger-counter works
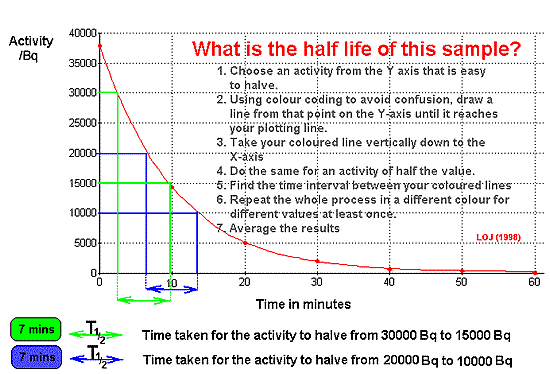
If the activity of
a sample is plotted against time, an exponential curve is obtained.
(NB
It must be the true activity - with the background count deducted
from each reading. If you are given a 'corrected count rate' that has
already been done for you!)
When plotting a graph, examiners like
to see candidates:
 Make maximum
use of the graph paper (choose the best scale - have paper orientated
the correct way so as to do this)
Label axes
with physical quantity and correct units
Make maximum
use of the graph paper (choose the best scale - have paper orientated
the correct way so as to do this)
Label axes
with physical quantity and correct units
 Mark the
points clearly. A neat cross is better than a 'blob'.. most computer
programs go for 'blobs'.
Mark the
points clearly. A neat cross is better than a 'blob'.. most computer
programs go for 'blobs'.
 Draw the
line of best fit. If the points indicate a curve, it should be
smooth (not 'dot-to-dot' like in a puzzle book). If they indicate
proportionality the line should be drawn with a ruler.
Draw the
line of best fit. If the points indicate a curve, it should be
smooth (not 'dot-to-dot' like in a puzzle book). If they indicate
proportionality the line should be drawn with a ruler.
 Work should
be neat (sharp pencil, long ruler, axes in ink etc.)
Work should
be neat (sharp pencil, long ruler, axes in ink etc.)
When analysing data
from a graph (whether drawn by them or given to them) candidates must
clearly show how the graph was used.
 When dealing with radioacive materials inside a human body we have to look at the effective half life, rather than just the physical one.
When dealing with radioacive materials inside a human body we have to look at the effective half life, rather than just the physical one.

 You may want to use a simulation instead of real sources - See here.
You may want to use a simulation instead of real sources - See here.




 It is a useful rule
of thumb to know that the activity of a sample drops to less than 1% of
its value in seven half lives (see
It is a useful rule
of thumb to know that the activity of a sample drops to less than 1% of
its value in seven half lives (see 
 See here
See here



