Uses of Nuclear Radiation

 Academic Applications - click here
Academic Applications - click here
 Industrial Applications - click here
Industrial Applications - click here
 Medical Applications - click here
Medical Applications - click here
 Other Applications - click here
Other Applications - click here
|
Alpha
|
Beta
|
Gamma
|
Positron
|
|
Short
half life
 Tracers
in industry - detecting leaks in pipes Tracers
in industry - detecting leaks in pipes
 Tracers in botony experiments - e.g. phosphorus 32 is a beta emitter - taken up by the plant - can be detected outside the plant as beta penetrates thin plant structures easily - half life of 14 days makes it ideal for this. Tracers in botony experiments - e.g. phosphorus 32 is a beta emitter - taken up by the plant - can be detected outside the plant as beta penetrates thin plant structures easily - half life of 14 days makes it ideal for this.
|
Short
half life
 Medical
tracer - used with gamma camera Medical
tracer - used with gamma camera
 Tracers
in industry - detecting routes of underground rivers and streams Tracers
in industry - detecting routes of underground rivers and streams
|
Short
half life
 Medical
tracer - PET Scanning Medical
tracer - PET Scanning
|
|
Long
half life
 Dating
of rocks using Uranium-238/lead ratios Dating
of rocks using Uranium-238/lead ratios
 Smoke
detectors Smoke
detectors
 Gas
lamp mantles Gas
lamp mantles
 Nuclear
batteries Nuclear
batteries
|
Long
half life
 Thickness
control of very thin metal sheets, paper or cardboard in manufacturing
and industry Thickness
control of very thin metal sheets, paper or cardboard in manufacturing
and industry
 C-14
dating C-14
dating
 Emergency
sign lighting Emergency
sign lighting
|
Long
half life
 High
activity - radiotherapy High
activity - radiotherapy
 High
activity - sterilisation of medical surgical instruments High
activity - sterilisation of medical surgical instruments
 High
activity - irradiation of food to kill bacteria and prolong
shelf life High
activity - irradiation of food to kill bacteria and prolong
shelf life
 Thickness
control of metal sheets (when too thick for beta) in manufacturing
and industry Thickness
control of metal sheets (when too thick for beta) in manufacturing
and industry
 Checking
welds Checking
welds
|
|
 Nuclear Batteries Nuclear Batteries
 The Apollo Moon missions used a radioisotope thermal generator (RTG). The NASA designation for the devices that powered the Apollo Lunar Surface Experiments Package (ALSEP) for missions 12, 14, 15, 16, and 17 was SNAP-27 (Systems for Nuclear Auxiliary Power model number 27). The energy source for this device was a rod of plutonium-238 weighing approximately 2.5 kilograms and providing a thermal power of approximately 1250W. Plutonium-238 is a non-fissile isotope of plutonium that decays by alpha particle emission with essentially zero associated gamma emissions. The Apollo Moon missions used a radioisotope thermal generator (RTG). The NASA designation for the devices that powered the Apollo Lunar Surface Experiments Package (ALSEP) for missions 12, 14, 15, 16, and 17 was SNAP-27 (Systems for Nuclear Auxiliary Power model number 27). The energy source for this device was a rod of plutonium-238 weighing approximately 2.5 kilograms and providing a thermal power of approximately 1250W. Plutonium-238 is a non-fissile isotope of plutonium that decays by alpha particle emission with essentially zero associated gamma emissions.
|

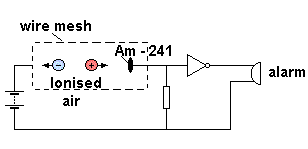 Smoke Detectors
Smoke Detectors
Some smoke detectors contain a small amount of Americium-241, an alpha emitter (and low energy gamma emitter) with a half life of 460 years. It consists of an ionisation chamber linked to a simple electronic alarm circuit The Americium ionises the air between the plates, causing a current to flow. Smoke entering the detector blocks some of the alpha particles, lowering the current, and triggering the alarm.
 Gas Lamp Mantles Gas Lamp Mantles
 Many camping lantern mantles used to contain thorium (alpha emitter with a long half-life see decay series). It apparently improved the flame. This practice has been stopped but old stock may still be around. Many camping lantern mantles used to contain thorium (alpha emitter with a long half-life see decay series). It apparently improved the flame. This practice has been stopped but old stock may still be around.
|
 Emergency Exit Sign Lighting Emergency Exit Sign Lighting
 During a fire, it's necessary to make sure that emergency exit signs remain illuminated, even if the power goes out. Some signs have a battery-powered light. Others have used tritium, a beta-emitting isotope of hydrogen, with a half-life of 12.3 years. During a fire, it's necessary to make sure that emergency exit signs remain illuminated, even if the power goes out. Some signs have a battery-powered light. Others have used tritium, a beta-emitting isotope of hydrogen, with a half-life of 12.3 years. |
 'Glow In The Dark'
Watches
'Glow In The Dark'
Watches
All radium dial watches should be disposed of properly. Above is a demonstration of the radioactivity from a radium-containing 1950's Timex watch dial, using Geiger counter.
They also use tritium,
see emergency exit signs above.
 Vaseline Glass Vaseline Glass
 Vaseline Glass is a particular color of yellow-green glass that is made by adding 2% Uranium Dioxide to the ingredients when the glass formula is made. The addition of the Uranium Dioxide makes the glass color yellow-green. It glows under ultraviolet rays. Vaseline Glass is a particular color of yellow-green glass that is made by adding 2% Uranium Dioxide to the ingredients when the glass formula is made. The addition of the Uranium Dioxide makes the glass color yellow-green. It glows under ultraviolet rays.
|
 'Glow In The Dark' Clock Hands 'Glow In The Dark' Clock Hands
 Radium was painted on the hands of clocks, so they would glow in the dark. The radium was painted by women, who had the bad habit of licking the brush tips to form them, ingesting the radium. This resulted in illness. See the MIT research document: Radium Dial Painting and Its Tragic Consequences Radium was painted on the hands of clocks, so they would glow in the dark. The radium was painted by women, who had the bad habit of licking the brush tips to form them, ingesting the radium. This resulted in illness. See the MIT research document: Radium Dial Painting and Its Tragic Consequences
|
LOJ
(February 2001) - revised March 2021



 Nuclear Batteries
Nuclear Batteries
 The Apollo Moon missions used a radioisotope thermal generator (RTG). The NASA designation for the devices that powered the Apollo Lunar Surface Experiments Package (ALSEP) for missions 12, 14, 15, 16, and 17 was SNAP-27 (Systems for Nuclear Auxiliary Power model number 27). The energy source for this device was a rod of plutonium-238 weighing approximately 2.5 kilograms and providing a thermal power of approximately 1250W. Plutonium-238 is a non-fissile isotope of plutonium that decays by alpha particle emission with essentially zero associated gamma emissions.
The Apollo Moon missions used a radioisotope thermal generator (RTG). The NASA designation for the devices that powered the Apollo Lunar Surface Experiments Package (ALSEP) for missions 12, 14, 15, 16, and 17 was SNAP-27 (Systems for Nuclear Auxiliary Power model number 27). The energy source for this device was a rod of plutonium-238 weighing approximately 2.5 kilograms and providing a thermal power of approximately 1250W. Plutonium-238 is a non-fissile isotope of plutonium that decays by alpha particle emission with essentially zero associated gamma emissions.  Smoke Detectors
Smoke Detectors Many camping lantern mantles used to contain thorium (alpha emitter with a long half-life see
Many camping lantern mantles used to contain thorium (alpha emitter with a long half-life see  During a fire, it's necessary to make sure that emergency exit signs remain illuminated, even if the power goes out. Some signs have a battery-powered light. Others have used tritium, a beta-emitting isotope of hydrogen, with a half-life of 12.3 years.
During a fire, it's necessary to make sure that emergency exit signs remain illuminated, even if the power goes out. Some signs have a battery-powered light. Others have used tritium, a beta-emitting isotope of hydrogen, with a half-life of 12.3 years.  Vaseline Glass is a particular color of yellow-green glass that is made by adding 2% Uranium Dioxide to the ingredients when the glass formula is made. The addition of the Uranium Dioxide makes the glass color yellow-gr
Vaseline Glass is a particular color of yellow-green glass that is made by adding 2% Uranium Dioxide to the ingredients when the glass formula is made. The addition of the Uranium Dioxide makes the glass color yellow-gr Radium was painted on the hands of clocks, so they would glow in the dark. The radium was painted by women, who had the bad habit of licking the brush tips to form them, ingesting the radium. This resulted in illness. See the MIT research document:
Radium was painted on the hands of clocks, so they would glow in the dark. The radium was painted by women, who had the bad habit of licking the brush tips to form them, ingesting the radium. This resulted in illness. See the MIT research document: 


