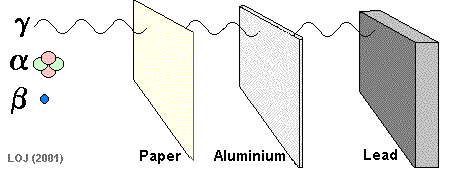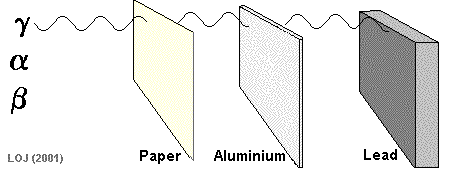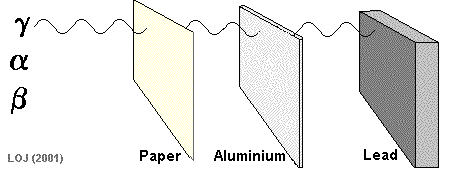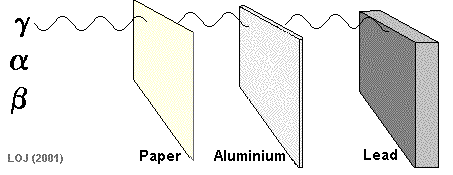
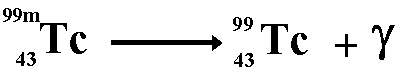Gamma Ray Emission The gamma ray
If a barrier is thick enough it will absorb most of the gamma rays that fall on it. Very few of the gamma rays emitted from the Sun reach the Earth's surface because the atmosphere is thick enough to absorb virtually all of them.
Production of Gamma RaysFor gamma ray emission to occur the nucleus must be in an excited state after emitting an alpha, beta or positron particle. Sometimes it stays like that for quite a while before the gamma ray is emitted (see metastable state), sometimes the emission of a gamma ray is instantaneous. Technicium 99m is a metastable form of technicium that is used widely in medical establishments.
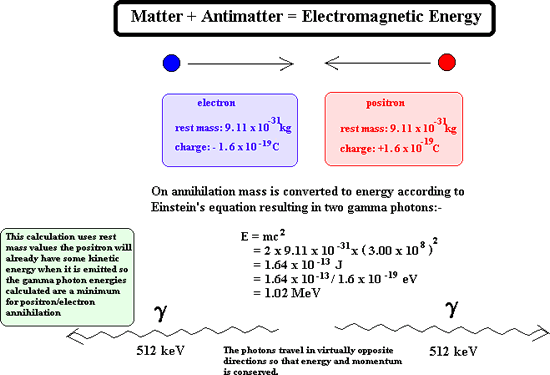
When gamma emission occurs there is no emission of matter particles therefore the nucleon number and the proton number remain the same. The remaining nucleus is of the same isotope but at a lower energy state. When antimatter meets with its matter counterpart annihilation occurs. Both particles disappear and two gamma rays are produced instead. For example:
Once emitted a positron does not get very far through matter before it reaches a 'matter electron' therefore its ionising power is great. As its ionising power is great it cannot penetrate very far at all into matter before it is annihilated. Its penetrating power is therefore very low indeed. However on interaction with matter it produces gamma rays and these have great penetration power.
|
Follow me...
|


 Having no mass and no charge its
Having no mass and no charge its