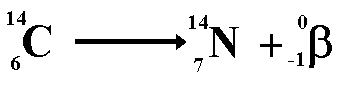|
||||
 Beta Particle Emission
Beta Particle Emission |
||||
|
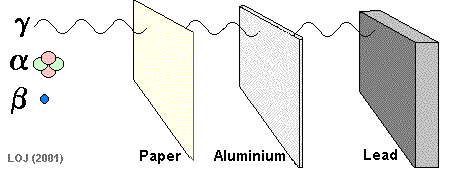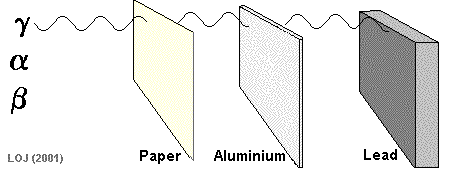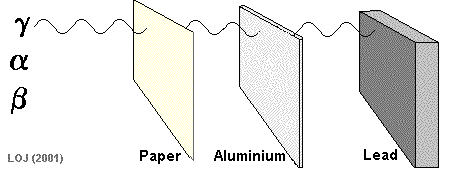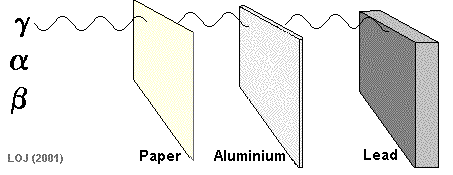
A beta particle It has a mass of 0.000055 u (see atomic mass unit) and a charge of +1.6 x 10-19 C Symbols: Being of such tiny mass (even on a nuclear scale) and having only a single negative charge its ionising power is low compared to the alpha particle. As its ionising power is low it can penetrate quite deeply into matter before its energy has been used up. Its penetrating power is therefore moderate (absorbed by 1m air, 0.1 mm lead or 3mm aluminium sheet).
Production of beta particles
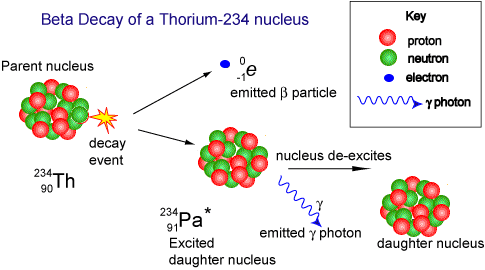
When beta decay occurs a neutron within the nucleus emits the particle and changes into a proton. Therefore the proton number increases but the nucleon number stays the same (only now you have one more proton and one less neutron!). The resulting daughter nucleus is of an element one position to the right of the 'parent' in the periodic table.
The animation below shows how the beta particle is formed from a neutron within the nucleus. The neutron becomes a proton (that remains in the nucleus) increasing the proton number (atomic number) of the nuclide, but leaving the nucleon number the same. The beta particle is emitted from the nucleus with very high kinetic energy.
|
||||
 |
||||

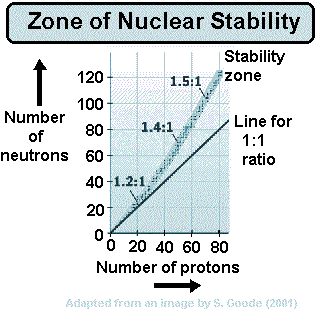 When a nucleus has too many neutrons to be stable, it tends to beta decay.
When a nucleus has too many neutrons to be stable, it tends to beta decay.