    |
||||

Electrostatic Charge |
||||
|
Outside of the nucleus of the atom there are only two fundamental forces acting in the physical world: the Graviational Force and the Electrostatic Force. The gravitational force governs the behaviour of mass and the electrostatic one governs the behaviour of charges! You are used to gravity because you have mass in 'your world' and it acts on that mass all of the time and you see the effects. You are not so aware of the electrostatic force because it only acts on you when you have a net charge - an electrostatic charge. Types of chargeThere are only two types of charge. They are opposite to each other and are called 'positive charge' and 'negative charge'. Charge is a special property that electrons and protons have. (At advanced level you will hear about other charged particles too - but you don't come across them until then!). Matter (all solids, liquids and gases) is made up of atoms. Atoms are composed of protons, neutrons and electrons. As atoms are made up of electrons and protons in equal number atoms the charge of an atom is zero - it is called a neutral atom.
Ions
The electrostatic force
Oppositly charged objects attract each other and like repel Charged objects experience a force from all other charged objects. The closer the object it the bigger the force experienced and the bigger the charges involved the stronger the force. Uncharged objects don't 'feel the force' at all! This force is called the electrostatic force. This electrostatic force is in addition to the force of gravity the object feels because of its mass. Therefore, if a charged object has very little mass the electrostatic force it experiences may be bigger than the gravitational force. If this electrostatic force acts in the opposite direction to gravity the object may be seen 'defying gravity'! Examples:
|
||||
 |
||||

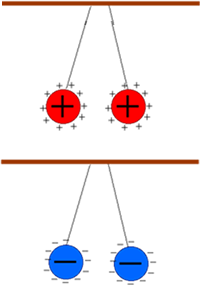
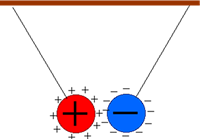
 When you charge up your body by touching the Van der Graaf generator your
hair stands on end. This is because the hair strands all gain the same
electric charge and repel each other. The force of repulsion is so great
that it exceeds the weight of each hair strand. Your arms do not lift
away from your body though - even though they have the same charge as
your body. This is because they are too heavy!
When you charge up your body by touching the Van der Graaf generator your
hair stands on end. This is because the hair strands all gain the same
electric charge and repel each other. The force of repulsion is so great
that it exceeds the weight of each hair strand. Your arms do not lift
away from your body though - even though they have the same charge as
your body. This is because they are too heavy! Here is a PowerPoint Presentation on Static Eectricity
Here is a PowerPoint Presentation on Static Eectricity
