|
An atom is the smallest chemically indivisible part of an element - that means you can't split it by chemical (or physical) means into anything smaller - it takes a nuclear reaction to do it!
All of the different types of atom known to exist in the universe are listed in the periodic table. Here is a simple diagram of the atom - you should KNOW this... including the sizes!
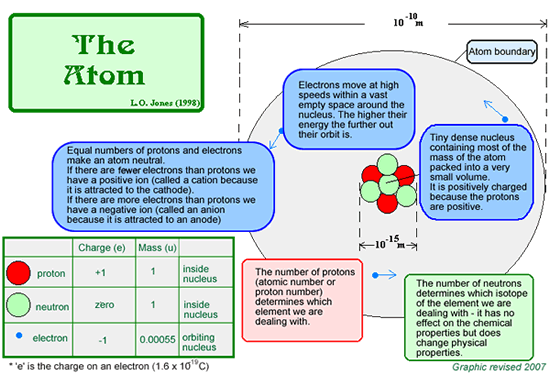
The above diagram is not to scale. If it was drawn to scale and you had a nucleus of 1 cm diameter in the centre of your screen you would need a screen of 1km diameter to show the full graphic! Most of the atom is empty space!.
Click here to find out how we know that the atom is like this.
 At KS4 you do not need to know the mass of the particles in the atom in kilograms. You just need to be able to compare the masses - to know that the mass of the proton is roughly the same as that of the neutron but that you would need almost 2000 electrons to have the same mass of electrons as that of a proton or neutron! At KS4 you do not need to know the mass of the particles in the atom in kilograms. You just need to be able to compare the masses - to know that the mass of the proton is roughly the same as that of the neutron but that you would need almost 2000 electrons to have the same mass of electrons as that of a proton or neutron!
 Never say the mass of an electron is zero - it is negligible but not zero - it can be rounded to zero because it is so small, but if you say it is zero in an examination you will get no marks! Never say the mass of an electron is zero - it is negligible but not zero - it can be rounded to zero because it is so small, but if you say it is zero in an examination you will get no marks! |
 At KS5 - A level - you are given the values on a data sheet and are expected to do calculations with them. At KS5 - A level - you are given the values on a data sheet and are expected to do calculations with them.
 |
This is a link to a jigsaw of the atom graphic - could be done on a whiteboard... or on your home PC.... it is a 'swf' so unfortunately not available on an iPad! |
 |
 Try this useful learning tool to help you learn this topic and/or test your knowledge... Try this useful learning tool to help you learn this topic and/or test your knowledge... |
 |
Ready for some questions?
Click on the icon - questions and answers at A level standard await you!
For more topics that have practice questions associated with them see the top menu bar - examination preparation
|
Click here to find out how we discovered the structure of the atom.
For Chemists among you here is an interactive illustration of the orbital shapes of electrons. Click here to find out more about the nucleus and click here for an overview of the atom from PPARC.
You may want to find out more about the atom. These links will take you to more advanced pages on my site:
 The nucleus The nucleus
 The electron orbitals The electron orbitals
 The Periodic Table The Periodic Table
|








 At KS4 you do not need to know the mass of the particles in the atom in kilograms. You just need to be able to compare the masses - to know that the mass of the proton is roughly the same as that of the neutron but that you would need almost 2000 electrons to have the same mass of electrons as that of a proton or neutron!
At KS4 you do not need to know the mass of the particles in the atom in kilograms. You just need to be able to compare the masses - to know that the mass of the proton is roughly the same as that of the neutron but that you would need almost 2000 electrons to have the same mass of electrons as that of a proton or neutron! Never say the mass of an electron is zero - it is negligible but not zero - it can be rounded to zero because it is so small, but if you say it is zero in an examination you will get no marks!
Never say the mass of an electron is zero - it is negligible but not zero - it can be rounded to zero because it is so small, but if you say it is zero in an examination you will get no marks!


 The nucleus
The nucleus
