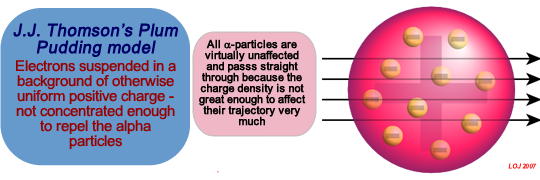
The Plum Pudding Model of the Atom The plum pudding model is a scientific 'model of the atom' proposed by J. J. Thomson in 1904. The model tried to explain what was known about the atom by scientists at the time. Atoms could not be 'seen', they were too small, but properties of atom were known at that time. It was known that:
His student Ernest Rutherford then came up with a series of experiments to test this idea for what an atom was like.
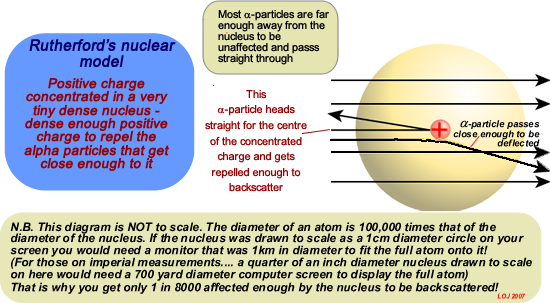
In 1909 Rutherford (by then a professor) then got his research students Geiger and Marsden to perform a series of experiments, expecting them to show that Thomson's model for the atom was correct. They fired alpha particles at gold atoms to find out how the electrons were arranged within the atom.This is what he expected to find:
But his students got some astounding results! Some alpha particles bounced back! They back-scattered or got deflected by very large amounts! Only a tiny number did this - 1 in 8000 - but after repeating and checking their results carefully Geiger and Marsden reported that it was happening!
The Plum Pudding model did not work - it could not explain that back-scattering! Rutherford came up with his model of the atom in 1911 after carefully looking at the reults of his research students' work. His model is very similar to the model we have today - a very dense central nucleus with positive charge orbited by negatively charged electrons.
|
Follow me...
|


 that they contained electrons - that were very tiny, negatively-charged particles of very low mass.
that they contained electrons - that were very tiny, negatively-charged particles of very low mass.  J.J. Thomson concluded that the most likely structure of the atom was that the atom was a uniformly positively charged region of space speckled with tiny negative electrons - giving an overall neutrality as long as none of the electrons left the atom 'cloud' of positive space. His 'plum pudding' model had electrons surrounded by a cloud of positive charge, like negatively-charged "plums" embedded in a positively-charged "pudding". The word "plums" in 19th-century English "plum pudding" is an archaic use of the word; it referred at that time to raisins, not plums as we think of them!
J.J. Thomson concluded that the most likely structure of the atom was that the atom was a uniformly positively charged region of space speckled with tiny negative electrons - giving an overall neutrality as long as none of the electrons left the atom 'cloud' of positive space. His 'plum pudding' model had electrons surrounded by a cloud of positive charge, like negatively-charged "plums" embedded in a positively-charged "pudding". The word "plums" in 19th-century English "plum pudding" is an archaic use of the word; it referred at that time to raisins, not plums as we think of them!