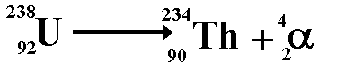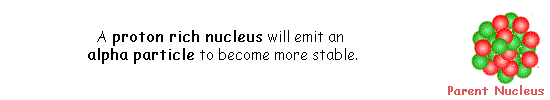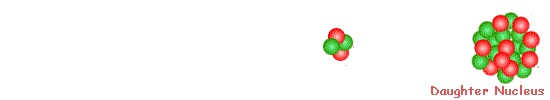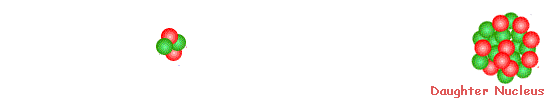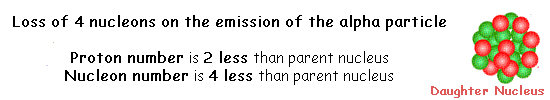|
||||
 Alpha Particle Emission
Alpha Particle Emission |
||||
|
An alpha It is therefore a helium nucleus It therefore has a mass of 4.0 u (see atomic mass unit)
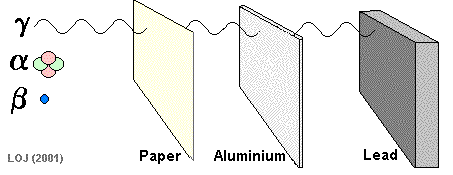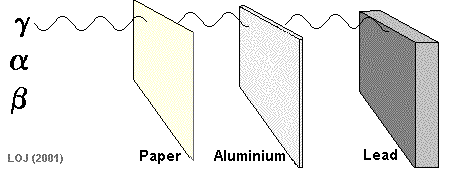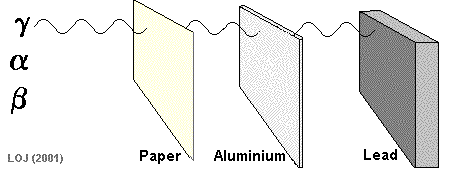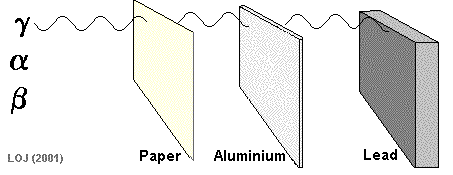
Being massive (on a nuclear scale!) and having a double positive charge its ionising power is high and its penetrating power is low. Alpha particles emanating from an alpha source would be absorbed by a sheet of paper.
Production of alpha particles
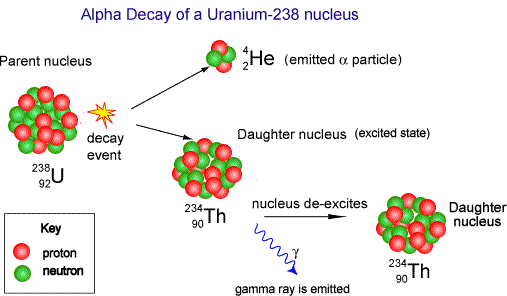
Decay is spontaneous and random. You cannot 'make' an atomic nucleus decay and you cannot predict when it will decay. All you can do is predict how many nuclei within a large number will decay in a given time interval. You can only give a probablity of decay occurring. When alpha decay occurs a group of two protons and two neutrons (a helium nucleus) comes out of the nucleus. Therefore the proton number decreases by 2 but the nucleon number decreases by 4.
The resulting daughter nucleus is of an element two positions to the left of the 'parent' in the periodic table.
|
||||
 |
||||

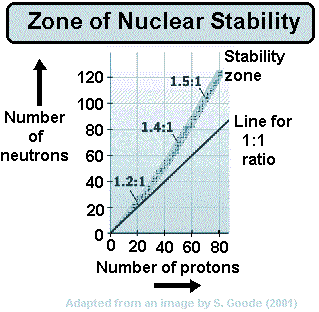 A nucleus that has high mass and too many protons to be stable tends to undergo alpha decay.
A nucleus that has high mass and too many protons to be stable tends to undergo alpha decay.