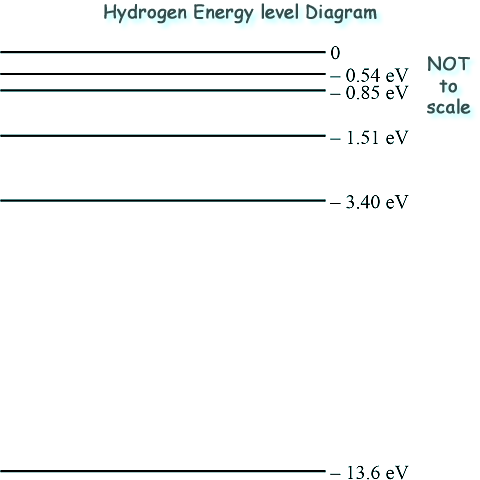
Quantum Phenomena - discrete energy levels for electrons Q7. In a discharge tube a high potential difference is applied across hydrogen gas contained in the tube. This causes the hydrogen gas to emit light that can be used to produce the visible line spectrum as shown below.
The visible line spectrum above has been used to predict some of the electron energy levels in a hydrogen atom. The energy levels predicted from the visible line spectrum are those between 0 and –3.40 eV in the energy level diagram. (a) Some of the predicted energy levels are shown below:
[3 marks]
[1 mark]
[1 mark]
[1 mark] (b) Discuss how the discharge tube is made to emit electromagnetic radiation of specific frequencies. In your answer you should:
[6 marks] (Total 12 marks) |
Follow me...
|