'A' Level Atomic StructureMultiple Choice Questions Q1. An atom of What is the specific charge of the ion?
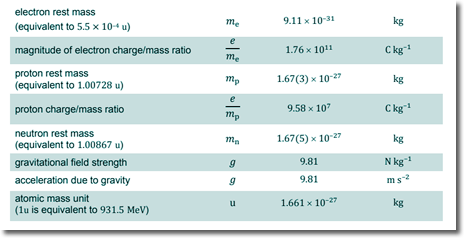
specific charge = Q/m charge on the ion = -3e Q = -3 x 1.60 x 10-19 C Q = - 4.80 x 10-19 C mass of the ion = 16 x 1.67 x 10-27 kg m = 2.67 x 10-26 kg specific charge = - 4.8 x 10-19/2.67 x 10-26 = - 1.8 x 107 C/kg (Choice B) Q2. Fluoride ions are produced by the addition of a single electron to an atom of fluorine What is the magnitude of specific charge of the fluoride ion?
specific charge = Q/m charge on the ion = -e Q = -1.60 x 10-19 C mass of the ion = 19 x 1.67 x 10-27 kg m = 3.2 x 10-26 kg specific charge = - 1.6 x 10-19/3.2 x 10-26 = - 5.0 x 106 C/kg
(Choice C)
Q3. The Rutherford scattering experiment led to:
|
Follow me...
|