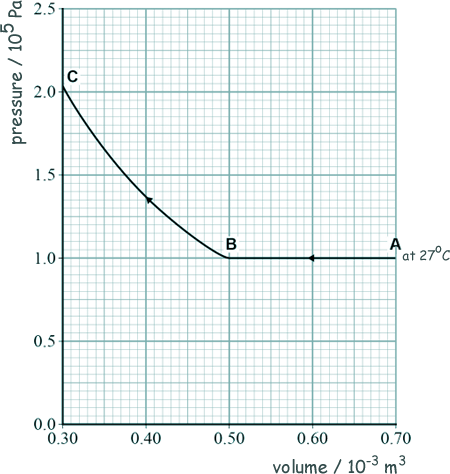
A level: Kinetic Theory Questions Q17. A number of assumptions are made when explaining the behaviour of a gas using the molecular kinetic theory model. (a) State one assumption about the size of molecules. [1 mark] (b) The graph shows how the pressure changes with volume for a fixed mass of an ideal gas.
At A the temperature of the gas is 27 oC. The gas then undergoes two changes, one from A to B and then one from B to C.
[2 marks]
[2 marks]
[2 marks]
[3 marks] (10 marks total) |
Follow me...
|