GCSE Questions: Radioactivity
Q3.
(a) When atoms of uranium-238 ( ) decay they produce a radionuclide called thorium-234 (
) decay they produce a radionuclide called thorium-234 ( ).
Thorium 234 decays by emitting beta radiation.
).
Thorium 234 decays by emitting beta radiation.
(i) What does beta radiation consist of?
(high kinetic energy or fast moving) electrons 
(1 mark)
(ii) Thorium-234 decays to form protactinium-234 (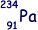 ).
).
What differences are there between the nucleus of a protactinium-234 atom and the nucleus of a thorium-234 atom?
Thorium has 90 protons within its nucleus whereas protactinium has 91 protons 
Thorium has 144 neutrons within its nucleus whereas protactinium has 143 neutrons 
(2 marks)
(b) The graph shows how the amount of radiation emitted by a sample of the radionuclide uranium-238 changes as time passes.
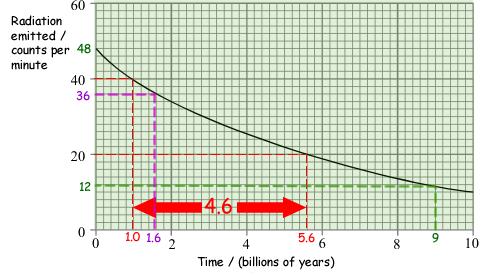
(i) Use the graph to find the half-life of uranium-238
Choose a value on the y axis - carry a dashed line to the graph line.
Find the half value of your choice - carry a dashed line to the graph line.
Calculate the time interval between them
The examiner looks for you to have marked a count rate and its half value on your graph. 
A mark goes for remembering to put in the unit.
An answer between 4.2- 4.8 obtained two marks as long as working was shown on the graph. 
(3 marks)
(ii) What fraction (or percentage) of the uranium-238 atoms will have decayed after 9 billion years?
In 9 billion years the count drops from 48 to 12 (see the green line on the graph) - it drops to 25% of the value, therefore 75% will have decayed ( 3/4)
(1 mark)
(c) Uranium-238 decays through a long series of intermediate radionuclides to stable atoms of the isotope lead-206 ( ).
).
A sample of igneous rock contains 3 atoms of uranium-238 for every atom of lead-206.
(i) The intermediate radionuclides are not important when estimating the age of the rock. Explain why.
The idea that the intermediate nuclides are relatively short-lived gains the mark. 
(1 marks)
(ii) Estimate the age of the rock. (You should explain how you obtained your answer).
3/4 of the rock is uranium - therefore only a quarter has decayed.
48 counts per minute will drop to 36 (see magneta lines on the graph)
1.6 billion years will have passed in that time. The rock is 1.6 billion years old.
(3 marks)
(Total 11 marks)


