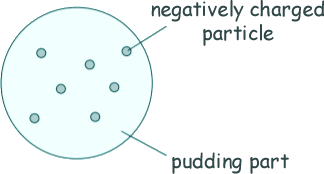
GCSE Questions: Nuclear Structure Q2. Over 100 years ago, scientists thought the atom was like a 'plum pudding'. The diagram shows the plum pudding model of the atom.
(a) The scientists knew that an atom has negatively charged particles. They also knew that an atom has no overall charge. What did the scientists conclude about the charge on the 'pudding part' of the atom? [1 mark] (b) Two scientists named Rutherford and Marsden devised an experiment to investigate the plum pudding model of the atom. The experiment involved firing alpha particles at a thin sheet of gold. The scientists measured how many of the alpha particles were scattered. Using the plum pudding model, the scientists predicted that only a few of the alpha particles would be scattered by more than 4°. Over several months, more than 100 000 measurements were made.
[2 marks]
[1 mark] (c) Describe the model now used for the structure of an atom. In your answer you should:
Do not include a diagram in your answer. [6 marks] (Total 10 marks) |
Follow me...
|