GCSE Questions: Nuclear Structure
Q2. Over 100 years ago, scientists thought the atom was like a 'plum pudding'. The diagram shows the plum pudding model of the atom.
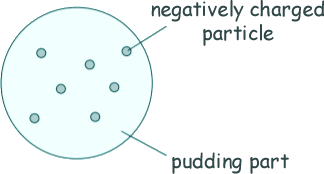
(a) The scientists knew that an atom has negatively charged particles. They also knew that an atom has no overall charge.
What did the scientists conclude about the charge on the 'pudding part' of the atom?
That it must have an equal amount of positive charge to cancel out the sum of the negative charge from the negatively charged particles. 
[1 mark]
(b) Two scientists named Rutherford and Marsden devised an experiment to investigate the plum pudding model of the atom.
The experiment involved firing alpha particles at a thin sheet of gold. The scientists measured how many of the alpha particles were scattered.
Using the plum pudding model, the scientists predicted that only a few of the alpha particles would be scattered by more than 4°.
Over several months, more than 100 000 measurements were made.
(i) The results from this experiment caused the plum pudding model to be replaced by a new model of the atom. Explain why.
As if the plum pudding model was correct it was expected that the alpha particles would go straight through, only slightly deflected from their path. But a significant number of alpha particles were scattered by more than 4o and alpha particles were even deflected backwards (back-scattered)  , these results could not be explained by 'plum pudding' model.
, these results could not be explained by 'plum pudding' model. 
[2 marks]
(ii) Suggest one reason why other scientists thought this experiment provided valid evidence for a new model of the atom.
Many/(over)100 000 measurements/results taken over several months
OR Rutherford(and Marsden) were respected scientists 
[1 mark]
(c) Describe the model now used for the structure of an atom.
In your answer you should:
 give details of the individual particles that make up an atom
give details of the individual particles that make up an atom
 include the relative masses and relative charges of these particles.
include the relative masses and relative charges of these particles.
Do not include a diagram in your answer.

| Level 3: |
A more detailed description is given, naming the particles and polarity of charge
and
either the relative mass is given for at least two particles
or the relative charge is given for at least two particles |
5 - 6 marks |
| Level 2: |
A description is given with all three particles named
and
either the polarity of charge associated with the three particles
or the relative mass of the three particles
or the relative mass for one particle and the relative charge for one particle given |
3 - 4 marks |
| Level 1: |
A brief description is given with some particles correctly named. |
1 - 2 marks |
| Level 0: |
No relevant information |
0 marks |
Examples of physics points that should be made in the response:
Brief description
 contains protons, neutrons and electrons
contains protons, neutrons and electrons
 protons are positive
protons are positive
 electrons are negative
electrons are negative
 neutrons are uncharged
neutrons are uncharged
 has a nucleus
has a nucleus
 relative charge: proton +1; electron – 1; neutron 0
relative charge: proton +1; electron – 1; neutron 0
 relative mass: proton 1; neutron 1; electron (about) 1/2000
relative mass: proton 1; neutron 1; electron (about) 1/2000
More detailed description
 protons and neutrons make up the nucleus
protons and neutrons make up the nucleus
 electrons orbit the nucleus
electrons orbit the nucleus
 electrons are in shells
electrons are in shells
 most of the atom is empty space
most of the atom is empty space
 nucleus occupies a very small fraction of the volume of the atom
nucleus occupies a very small fraction of the volume of the atom
 electrons orbit at a relatively large distance from the nucleus
electrons orbit at a relatively large distance from the nucleus
 most of the mass of the atom is contained in the nucleus
most of the mass of the atom is contained in the nucleus
 the nucleus as a whole is positively charged
the nucleus as a whole is positively charged
 total number of protons in the nucleus equals the total number of electrons orbitting it in an atom
total number of protons in the nucleus equals the total number of electrons orbitting it in an atom
[6 marks]
(Total 10 marks)







