|
Metals 
The structure of metals The arrangement of the atoms Metals are giant structures of atoms held together by metallic bonds. "Giant" implies that large but variable numbers of atoms are involved - depending on the size of the of metal piece.
The electrons can move freely within molecular orbitals that are formed between atoms and so each electron becomes detached from its parent atom. The electrons are said to be delocalised. The metal is held together by the strong forces of attraction between the positive nuclei and the delocalised electrons. This is rather like a neighbourhood parent network that allows children to move between homes within that network - the electrons have an 'extended family network' rather than being held onto tightly by one parent atom!
There are also 3 atoms touching any particular atom in the layer above and another 3 in the layer underneath. This second diagram shows the layer immediately above the first layer. There will be a corresponding layer underneath. The electrons therefore have a three dimensional molecular bond area - sea of delocalised electrons in which they can flow. Crystal grains It would be misleading to suppose that all the atoms in a piece of metal are arranged in a regular way. Any piece of metal is made up of a large number of "crystal grains", which are regions of regularity At the grain boundaries atoms have become misaligned causing irregularity within the structure. Even within a crystal grain you get rather subtle irregularities known as 'dislocations'. This is important when we consider placing stress on metal objects and explains on a microscopic level what can happen when a metal is put under stress. The physical properties of metals Melting points and boiling points Metals tend to have high melting and boiling points because of the strength of the metallic bond. The strength of the bond varies from metal to metal and depends on the number of electrons which each atom delocalises into the sea of electrons, and on the packing. Electrical conductivity Metals conduct electricity very easily and have high conductivity - low resistivity. The delocalised electrons are free to move throughout the structure in 3-dimensions. They can even cross grain boundaries. Even though the pattern may be disrupted at the boundary, as long as atoms are 'touching' each other, the metallic bond is still present. Liquid metals also conduct electricity, showing that although the metal atoms may be free to move, the delocalisation remains in force until the metal boils. Gaseous metals do not conduct electricity. Thermal conductivity Heat energy can be transferred by the electrons as excess kinetic energy (it makes them move faster) as well as via lattice vibrations by the heat transfer method of conduction.. The energy is transferred rapidly throughout the rest of the metal by the moving electrons. That is why metals are such good conductors of heat. Strength and workability Malleability and ductility Metals are described as malleable (can be beaten into sheets) and ductile (can be pulled out into wires). This is because of the ability of the atoms to roll over each other into new positions without breaking the metallic bond.
Elastic deformation is a change in shape of a material at low stress that is recoverable after the stress is removed. This type of deformation involves stretching of the bonds, but the atoms do not slip past each other.
Study of the streching of metals is covered under the Young Modulus and Hooke's Law.
|
Follow me...
|


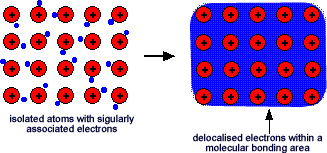 This is sometimes described as "an array of positive ions in a sea of electrons".If you are going to use this view, beware! Is a metal made up of atoms or ions? It is made of atoms - the metal atom has not let go of its outermost electron - it has just allowed it to wander freely.
This is sometimes described as "an array of positive ions in a sea of electrons".If you are going to use this view, beware! Is a metal made up of atoms or ions? It is made of atoms - the metal atom has not let go of its outermost electron - it has just allowed it to wander freely. Most metals are close packed - that is, they fit as many atoms as possible into the available volume. Each atom in the structure has 12 touching neighbours. Such a metal is described as 12-co-ordinated.
Most metals are close packed - that is, they fit as many atoms as possible into the available volume. Each atom in the structure has 12 touching neighbours. Such a metal is described as 12-co-ordinated. Each atom has 6 other atoms touching it in each layer.
Each atom has 6 other atoms touching it in each layer. If a small stress is put onto the metal, the layers of atoms will start to roll over each other. If the stress is released again, they will fall back to their original positions. Under these circumstances, the metal is said to be suffering elastic deformation - see
If a small stress is put onto the metal, the layers of atoms will start to roll over each other. If the stress is released again, they will fall back to their original positions. Under these circumstances, the metal is said to be suffering elastic deformation - see 


