Technetium 99m Nuclear medicine is the branch of medicine that uses radioisotopes for research diagnosis and treatment of disease. Since its humble beginnings in 1958 (see here for details of how it was discovered), technetium-99m has become the most widely used radioisotope for diagnosing diseased organs. All isotopes of technetium are radioactive.Technetium-99m is a metastable isotope of the element technetium. It's short half life means that it is not found in any abundance naturally and therefore it has to be produced in a nuclear reactor. It is classed as a man-made or artificial isotope rather than naturally occurring. The name originates from the Greek word 'technetos' meaning artificial! The common isotope of technetium is Tc-98, but it is the metastable isotope of Tc-99 that is most sought after - Tc-99m. The 'm' in its name stands for metastable. That means it has an excited nucleus. In order for that nucleus go to a more stable state it has to give out the excess energy, it does this by emitting a gamma ray. Tc-99 m is ideal as a medical tracer because the gamma radiation it emits allows the medical practitioner to image internal body organs causing hardly any radiation damage to the patient. Technetium-99m is used in over 20 million diagnostic nuclear medical procedures every year, half of which are bone scans, and the other half are roughly divided between kidney, heart and lung scans. Approximately 85% of diagnostic imaging procedures in nuclear medicine use this isotope. Tc-99 m is an ideal marker radionuclide for imaging as it is:-
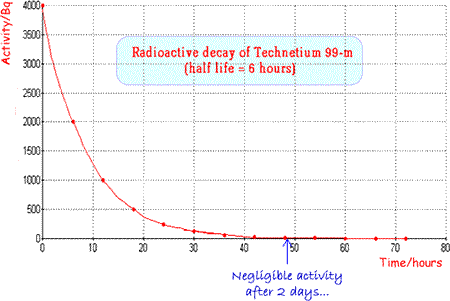
Technetium 99m is a very useful radionuclide in gamma imaging because it only emits gamma rays and these are of an energy that are easily detected by a gamma camera (140keV). It has an ideal half life (six hours) which is long enough for diagnostic procedures to take place but short enough for the patient not to be inconvenienced unduly by remaining ‘radioactive’ for too long a period after the investigation. It is suitable not only for use alone but also for attachment to a wide range of compounds for tracer experiments. It can easily be produced ‘in situ’ using a ‘cow’ as it is the daughter nucleus of the decay of Molybdenum 99 and is easily separated from the parent (more hazardous b-emitter) by a saline flush. Molybdenum-99 has a half-life of 66 hours, allowing it to be transported over fairly long distances. A fresh supply is brought to the hospital fortnightly and then the ‘cow’ is ‘milked’ as and when required. When Technetium 99m is used as a radioactive tracer a 'butterfly' can be used to ensure that an injection of radioactive material is inserted into the correct site. |
Follow me...
|