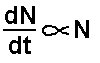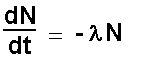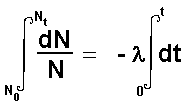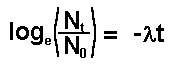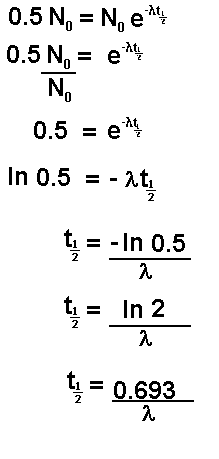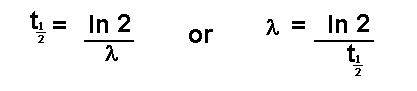Radioactive
Decay at 16+
All radioactive decay occurs in such a way as the
number of atoms 'decaying' in unit time (dN/dt) is directly proportional
to the number of undecayed radioactive atoms present in the sample at
that time (N).
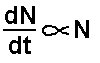
The mathematics of statistics applies (a very large
number of 'events' needs to be considered). A constant of proportionality
l (called
the decay constant) replaces the proportionality symbol, thereby making
the equation simpler:
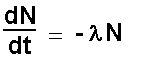
With time the number of undecayed atoms decreases.
The activity decreases therefore the change in activity is negative.
Adding the negative sign allows us to have a positive value for the
activity. Note the negative sign!
The type of equation is called a differential equation.
Calculus can be used to integrate this giving and expression that incorporates
both the initial number of atoms present and the final number present
after a time interval 't'
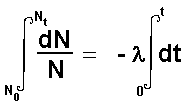
When solution of this equation is:
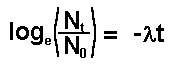
Therefore
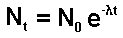
Thus we have a mathematical expression that shows
that radioactive decay is exponential.
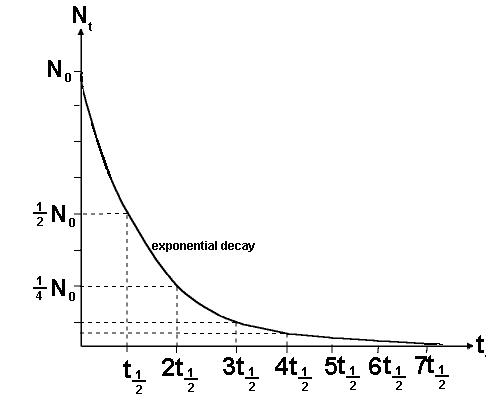
If N
t is half of N
0 then the
time t is the time taken for half of the sample to undergo spontaneous
disintegration or decay. This is termed the half life and is given the
symbol
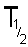 or
or 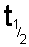
Let us follow the mathematics of this through
for a half life value:
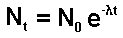
Now let us insert the number of atoms present in
terms of the original number:
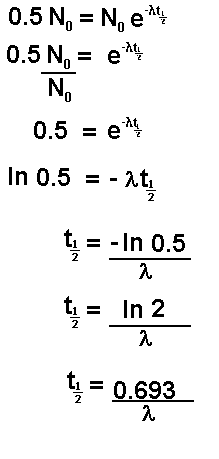
How this is achieved should be understood. If necessary
look in the
mathematics
section at the background on use of
logs.
It is important that l can be found directly from
the half life and vice versa is remembered.
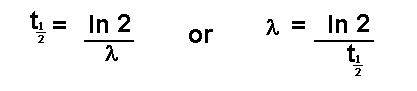
A similar calculation can be done to find the time
taken for a certain percentage of a sample to decay.
Try the following question:
The half-life of cobalt 61 is 100 minutes.
How long does it take for the activity of the sample to fall to
80% of its initial value?
or to find the percentage left after a certain time
lapse
Try this question:
The half-life of radioactive zinc is
50 hours. Find the percentage loss of activity which occurs for
a sample each hour.
Now use
the interactive
spreadsheets to investigate the half life idea in a little
more depth...