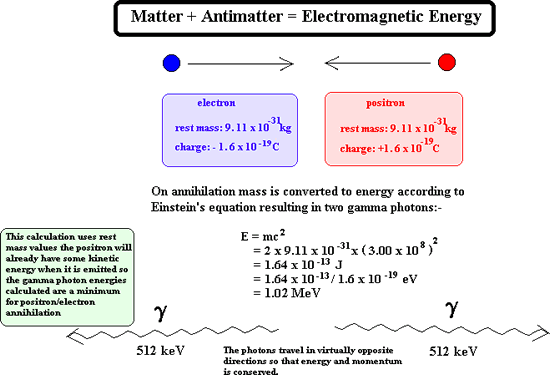
Positron Emission A positron It is a particle of nuclear radiation that is an anti-electron of high kinetic energy. It has a mass of 0.000055 u (see atomic mass unit) Symbols: It has a charge of +1.6 x 10-19 C (same magnitude but opposite in sign to an electron) When it meets with an electron it annihilates it. Both particles disappear and two gamma rays are produced instead.
Once emitted a positron does not get very far through matter before it reaches a 'matter electron' therefore its ionising power is great. As its ionising power is great it cannot penetrate very far at all into matter before it is annihilated. Its penetrating power is therefore very low indeed. However on interaction with matter it produces gamma rays and these have great penetration power. Production of positrons
When positron decay occurs a proton within the nucleus emits the particle and changes into a neutron. Therefore the proton number decreases but the nucleon number stays the same (only now you have one more neutron and one less proton). The resulting daughter nucleus is of an element one position to the left of the 'parent' in the periodic table. The animation below shows how the positron is formed from a proton within the nucleus. The proton becomes a neutron (that remains in the nucleus) thereby decreasing the proton number (atomic number) of the nuclide, but leaving the nucleon number the same. The positron is emitted from the nucleus with very high kinetic energy.
|
Follow me...
|


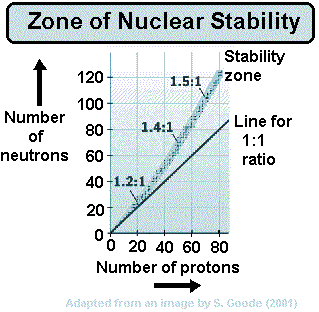 When a nucleus has too many protons to be stable, it tends to positron decay.
When a nucleus has too many protons to be stable, it tends to positron decay. 




