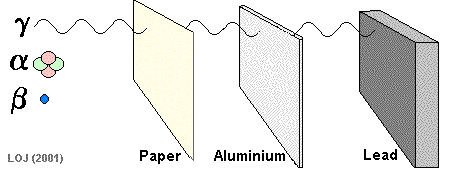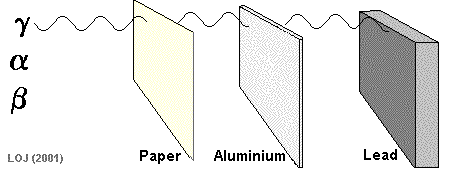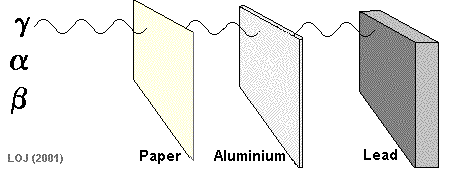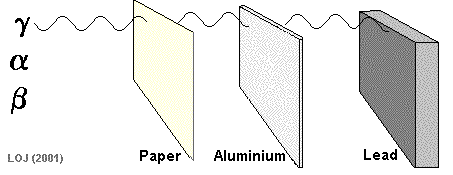
Ionizing Power and Penetrating Power Ionizing Power and Penetration Power are linked. The penetrating power of nuclear radiation depends upon the ionising power of the radiation. The radiation continues to penetrate matter until it has lost all of its energy. The further it can penetrate into the substance the more spread out the ionization it causes will be, so the more localised the ionization the less penetrating power it will possess. Alpha
particles are the least penetrating as they are the most densely
ionizing. They are absorbed by 10 cm of air, 0.01 mm lead or a sheet
of paper. This
means that if a given number of alphas are fired at a target they
will all cause ionization near the surface of the material, resulting
in the effects of the radiation being concentrated in a small volume.
The double charge and considerable mass of the alpha in comparison
with the other nuclear radiation forms explains why the impact on
matter is so great.
The more localized the damage in living tissue the greater the chance of mutations occuring. DNA is the material within cells that carries the information about the living creature. DNA molecules are long helical strands with four different types of branches coming off the stem. If these branches are altered in any way the biological 'code' is altered and the cell may reproduce itself 'wrongly'. This can lead to tumors in the living being itself and/or mutated offspring if reproductive cells are affected. Bilological systems can cope with one or two 'faults', detect them and deal.... but if the damage is highly localized there may be too many and defective cells may reproduce and cause problems. Therefore the highly localized damage caused by alpha particles is most dangerous. If the alpha source is outside the body the alpha particles would all be absorbed by the outer (dead) layer of skin cells... but if it is within the body it could cause damage to vital organs. That is why powdered sources of alpha emitters are highly dangerous. They can be inhaled and lodge sources of alpha particles deep in the lungs, or swallowed and get lodged in the digestive system etc.Tiny grains of a radioactive source can contain millions of atoms. Beta sources are less likely to cause as much extensive damage in a localised area as an alpha source of the same activity, but the risk of mutation is still there and all radiation sources are dangerous. Gamma
sources lodged inside your body would send out gamma rays through
you. Some would interact with your body - so they ARE dangerous
to you, but most would get straight out and make you a source of
radiation to those around you! These are the only radioactive sources
that it is worth the risk of putting inside a patient for diagnostic
reasons - see the gamma
camera. |
Follow me...
|