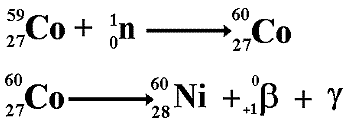
Artificial Transmutation of the Elements - the Production of Manmade Radioisotopes
With the advent of nuclear reactors that is no longer just a dream! It is called artificial transmutation of elements and it has allowed us to 'make' elements that might have never before existed on our planet - the transuranic elements! Nuclear fission reactors are not just a source of heat for power production. They are also an abundant source of neutrons. As neutrons have no charge, they have the ability to be inserted into the nuclei of a wide variety of elements. This usually makes the nucleus 'neitron rich' and makes the nucleus unstable. It therefore makes the material that is bombarded with neutrons into a radioactive isotope of the original material. The radioactive nuclei will then decay into nuclei of another element. Canada as one of the primary sources of isotopes for medical and industrial use because the type of nuclear reactor they have built there (light water reactors) makes nuclear transmutation easier to do. They therefore make the isotopes and sell them around the world.
This process is also the reason why equipment used in the reaction chamber of a nuclear reactor become radioactive. It also explains why the shielding surrounding the chamber also becomes radioactive - that is because of the array of radioactive isotopes that make up its structure after bombardment with neutrons (not because it has become 'contaminated' with isotopes from within the chamber - as many students mistakenly believe!). Transuranic ElementsThese are elements with proton numbers greater than 92 (the proton number for Uranium!). It is only since the dawn of the nuclear age that elements with proton numbers bigger than 92 have existed on our planet. The transuranic elements are not naturally occurring, they are artificially created. They are made by firing neutrons at the nuclei of heavy atoms such as uranium. The nucleus absorbs the neutron and then becomes 'neutron rich' and decays by beta emission to produce atoms with bigger proton numbers. Once the isotopes become 'proton rich' they then decay by alpha emission thereby reducing the proton number and mass.
If you are interested in the history of the production of transuranic elements take a look at this page. It lists their discovery and gives the background to thier names. |
Follow me...
|



 Alchemists dreamt of changing one element into another.
Alchemists dreamt of changing one element into another.