Strong Nuclear Force
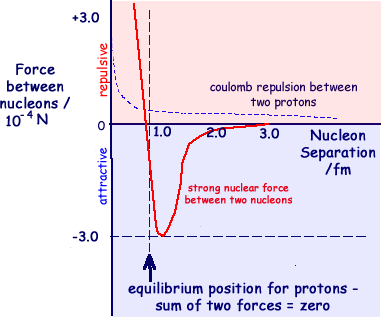
The strong nuclear interaction is the mechanism responsible for this force and the color force is the force (between quarks) that is responsible for this interaction. The exchange particle for the strong interaction is the gluon. The graph below shows how the strong nuclear force between nucleons varies as the separation of the nucleons increases. It also shows how the repulsion between two protons varies according to the electromagnetic force.
Notice that:
When researching this topic I found some conflicting information! This was particularly so concerning equilibrium distances. I suggest that A level candidates quote the values given in the syllabus they are studying. AQA Syllabus quote: 'The strong nuclear force; its role in keeping the nucleus stable; short-range attraction to about 3 fm, very-short range repulsion below about 0.5 fm;' OCR Syllabus quote : 'short-range nature of the force; attractive to about 3 fm and repulsive below about 0.5 fm'
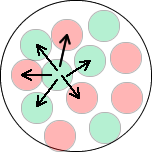
The range of the nuclear force is such that a nucleon only affects those nucleons next to it. With a range of just under 3 fm and the diameter of a single nucleon being about 1.75 fm, only nearest neighbours within a nucleus can 'feel the force'. The diagram indicates the protons (red) and neutrons (green) affected by one of the neutrons in the diagram. This 'affecting neighbours' results in the nucleus being pulled into a sphere of attracting nucleons occupying a space of a couple of femtometres diameter. The more nucleons associated together the bigger the volume - but the average distance between nucleons is the same no matter what the size of the nucleus - nuclear density is roughly constant because of the action of the strong nuclear force. See nuclear density. Electromagnetic repulsion compared with the strong force of attraction.The much smaller repulsive electromagnetic force between protons has a much larger range and becomes the only significant force between protons when their separation exceeds about 2.5 x 10-15m. The strong nuclear force is not connected with charge. Proton-proton, proton-neutron and neutron-neutron forces are the same. (The force between protons, however, must always be modified by the Coulomb repulsion between them - but when at the point where the strong force is great you would have to be calculating to over 3 significant figures to notice its affect as the electromagnetic force is 10-2 times the strong nuclear force.) |
Follow me...
|


 The force responsible for holding all nucleons together is called the strong nuclear force.
The force responsible for holding all nucleons together is called the strong nuclear force.