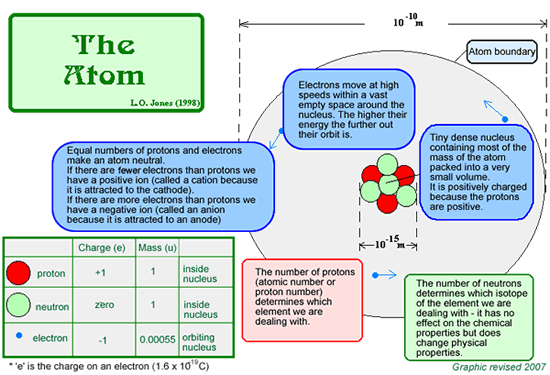
Nuclear Power at A Level - Basic Revision Summary Some atomic nuclei split into two parts (nuclear fission) - not usually equal ones but always into two - giving two smaller fission fragments. Not all atoms do this, only ones that are really unstable. Heat energy is released when an atom nucleus splits . Uranium-235 atoms can be made to undergo fission. They are a store of nuclear (or atomic) energy that we can use as a fuel. The heat produced is used commercially to produce electricity in nuclear power stations. The heat energy is used to boil water and the pressure of the steam produced is used to turn turbines which operate electrical generators and make electricity. Revision of basics:
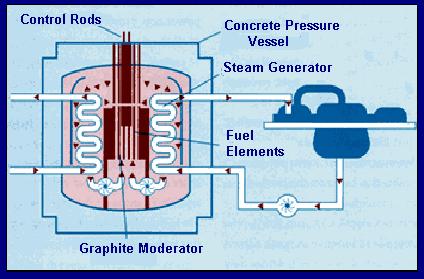
The splitting of the atomic nucleus (nuclear fission) produces a lot of energy.This can then be used to make electricity from the heat extracted from a nuclear reactor (by using it to boil water, producing steam, the pressure from which can be used to turn turbines (so that electricity can be produced by electromagnetic induction)). This is called nuclear power or atomic power. A nuclear Power PlantIn a nuclear power plant use is made of this fission reaction. A nuclear reactor is a device in which nuclear chain reactions are initiated, controlled, and sustained at a steady rate. The energy released is used to heat water, making steam, the pressure from which can be used to turnturbines and produce electricity (by electromagnetic induction ).
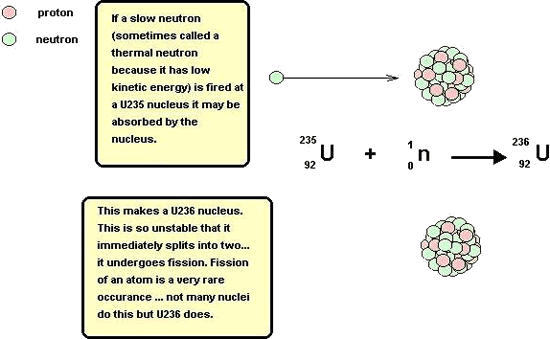
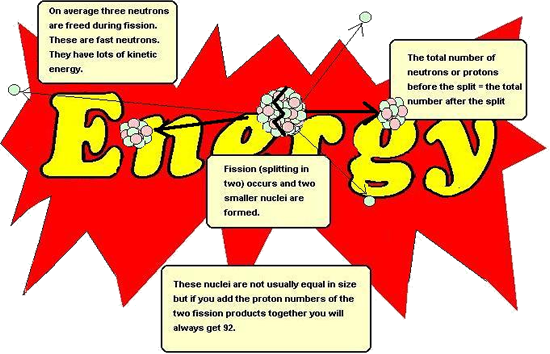
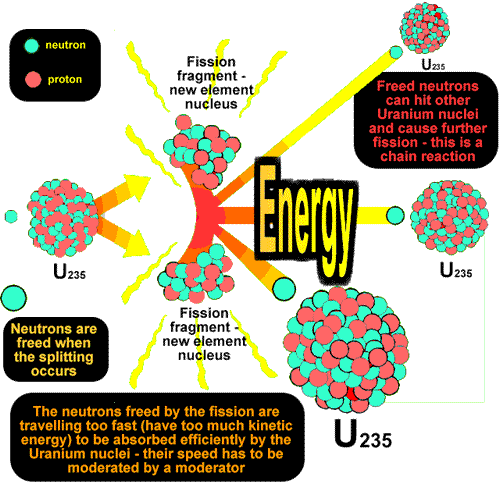
Chain reaction - using the neutrons made by fission to instigate a new fissionBecause a neutron is needed to instigate a fission (splitting) and neutrons are produced as the product of a fission, these products can go on to produce more fissions,the neutrons produced in which can go on to produce more fissions etc.etc. Each stage is therefore linked to the previous one and therefore is termed a chain reaction.
The only problem with the chain reaction is the fact that each stage of the reaction instigates more than twice as many fissions as the next stage (almost three times as many!).This results in the amount of energy produced at each stage more than doubling if the reaction is left unchecked. Each stage occurs in less than a millionth of a second therefore the heat energy produced can be phenomenal.The reaction is said to escalate. If we requirea steady energy output of energy only one neutron from each fission should be allowed to go on to make another fission reaction. The reaction needs to be controlled. Controlling the energy output of the reactorBoron is a very good absorber of neutrons. It is said to have 'a high cross section for neutrons'. Rods of boron (or cadmium - it has a high cross section for neutrons too) are lowered into the reaction-vessel enough to absorb a sufficient quantity of the neutrons produced by fission so that only about one of those produced in a split goes on to instigate a new fission. If more energy is required by the power station, the rods can be lifted out of the vessel (allowing more neutrons to cause fission). If les senergy is required they can be lowered deep into the vessel (mopping up more neutrons). If they are pushed right in, they can mop up enough neutrons to prevent the chain reaction from continuing; this will result in shutting down the reactor safely. These rods are called control rods because they control the energy output of the reactor. Moderating the speed of neutrons so that efficient fission occursEfficient fission occurs if the bombarding neutrons are likely to stay in the nucleus and cause the instability that results in a fission (split).This is only the case is they are of thermal energy (slow). The neutrons resulting from the fission are usually of too high a kinetic energy to interact with the nucleus successfully and therefore need their speed moderating before they will have a high probability of producing a fission. Graphite is therefore included in the reaction vessel. It is an excellent moderator.It takes energy out of the neutrons (thereby slowing them down) as they interact with the carbon atom lattice(3-d bonded structure of graphite) but is does not absorb too many of them. It is said to have a 'low cross-section for neutrons '. It is called a moderator because it moderates the speed of the neutrons. At A level you should be able to:
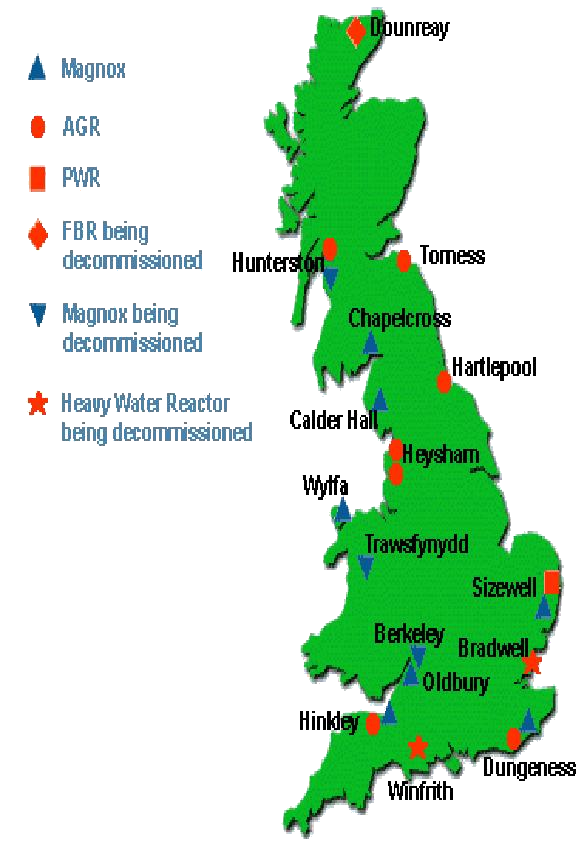
Power in the UKSeveral types of nuclear
reactor have been built in Britain. Commercial reactors commissioned between
1956 and 1973 were of the gas-cooled Magnox design. Between 1976 and 1988,
seven advanced gas-cooled reactors (AGRs) were built.
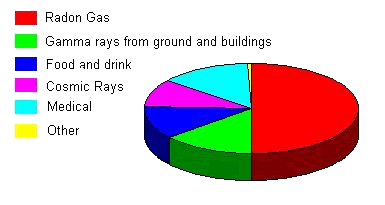
Safety ConsiderationsIrradiated fuel is highly radioactive and is kept carefully segregated. The radioactivity decays with time and initially the rate of decay is very rapid. Ultimately the spent fuel can be either placed in a long-term store, possibly for several decades, or transferred to a reprocessing plant at Sellafield, where reusable uraniumand plutonium is separated out from high level waste. A vitrification (converting the waste into a large glass block) plant at Sellafield enables reprocessed high level waste to be solidified in glass blocks, sealed in stainlesssteel cans and placed in a carefully controlled storage facility. In its 1995 Policy Review the Government concluded that underground disposal was the favoured option for the long-term management of vitrified high level waste and reported that it was putting in hand development of a research strategy, with the aim of producing a statement of future intent in this area. It also concluded that a repository for the disposal of low and intermediate level radioactive waste should be constructed as soon as reasonably practicable. In the meantime disposal of low level waste by shallow burial at the Drigg site in Cumbria would continue. Very low levels of radioactivity are released from nuclear plant in gaseous emissions via stacks or in liquid effluent discharged to the sea. These releases are kept below authorised limits set by the Environment Agencies, using a variety of techniques,such as filtration and ion-exchange treatments. These limits ensure thatthe levels of radioactivity released to the environment are negligible compared with natural background levels. The discharges are monitored by both the nuclear industry and the Ministry of Agriculture, Fisheries and Food and the results of the monitoring are published regularly. As a result of these controls, population radiation exposure due to discharges from a nuclear power station is about the same as that due to the emissions from a coal-fired station, arising from the tracesof natural radioactivity in the coal. Everyone is exposed to background radiation from the sun and outer space and fromthe natural radioactivity in rocks, soil, buildings and diet. Thesesources account for 85% of the average person's annual radiation dose andmost ofthe rest comes from medical sources such as X-rays.
The contribution from nuclear industry discharges amounts to less than 0.1% of the total. Over a year this amounts to less than the radiation dose received from eating one brazilnut. (These nuts naturally accumulate radioactive elements from the soilduring growth.) Despite the rigorous control of radioactive discharges from power stations, it has been suggested that these increase the risk of childhood leukaemia, and links have been claimed at some nuclear sites. This suggested association has been the subject of intensive research over the past decade. The latest authoritative study in England and Wales by Oxford University researchers, published in the British Medical Journal in 1994, used a very sensitive, new technique for detecting raised incidence of disease near a suspectedsource of risk. Whilst an excess of childhood leukaemia and related diseases near Sellafield was clearly apparent, the authors concluded that there was no evidence of a general increase of these diseases around nuclear installations. A similar conclusion was reached in a recent study in Scotland. Other possible explanations have been put forward to explain the excess near Sellafield, notably the Kinlen hypothesis that leukaemia is a rare response to a common infection whose spread is facilitated by population mixing, as in new towns, for example. Research is continuing worldwide into the causative mechanisms for human leukaemia. Environmental Benefits of Nuclear PowerIn its environmental white paper, This Common Inheritance, 1990 (Cm 1200), and in its strategies on Climate Change (Cm 2427) and Sustainable Development (Cm 2426) in 1994, the Government recognised that nuclear power made a major contribution to curbing acid rain and combating global warming. Nuclear stations emit negligible quantities of the acid rain gases, sulphur dioxide (SO 2)and nitrogen oxides (NOx), and the greenhouse gas carbon dioxide(CO2), a major contributor to global warming. Small amounts of these gasesare emitted as a result of uranium mining, fuel processing and transport, but this is negligible compared to the amount produced from fossil fuels. Table 1 compares the full fuel cycle emissions for the major fuels usedin generating electricity. Emissions
in grams per kWh delivered to final customer
* No flue gas desulphurisation
Source : Energy Technology Support Unit If all the electricity produced by nuclear stations in 1995 had been generated by coal stations, the UK'stotal CO2 emissions would have been 13%, or almost 20 milliontonnesof carbon (MtC), greater. Emissions of SO2 and NOx wouldalso have been much higher. From 1990 to 1994, the output from nuclearstations increased by 36%, thereby making CO2 emission savingsagainst generationfrom coal equivalent to over 5 MtC per annum. This isover half the 10 MtCreduction in annual national CO2 emissionsbetween 1990 and 2000,to which the UK is committed under the UN ClimateChange Convention. In Francethe large nuclear programme of the late 70sand 80s led to an 80% reductionin the annual CO2 emissionsfrom power stations within 7 years. Decommissioning and DisposalWhen a nuclear power stationcomes to the end of its useful life, decommissioning of the station commenceswith the aim of eventually returning the site to alternative uses with no nuclear legacy. Dismantling of the stationtakes place in stages, taking full account of public safety in containingthe radioactivity at all times. The nuclear companies' "safestore" decommissioningstrategyprovides for reactor defuelling immediately after shutdown, taking 2-3 years and removing 99.9% of the residual radioactivity. Demolition of non-radioactive plant and buildingsand dismantling of some radioactive plant is then undertaken. This is followed by the safe and secure maintenance of remaining structures for up to 135 years before final dismantling and site clearance.This ensures that radiation has fallen to a safe working level; it alsoreduces the amount of radioactive waste. In its Radioactive Waste Management Policy Review, the Government acknowledged the suitability of the safestore strategy. It required operators to submit their decommissioning proposals every five years for review by the Health and Safety Executive, in consultation with the Environment Agencies. Whilst recognising that the nuclear companies were in the process of making full provision for decommissioning in their accounts, the Review nevertheless required the establishment ofsegregated decommissioning funds for the privatised parts of the industry. The first commercial UK reactor to be closed for decommissioning was the Magnox station at Berkeley. Decommissioning work started in 1989 and is progressing to the care and maintenance stage. The Magnox stations at Trawsfynydd in Snowdonia and at Hunterston in Scotland are also being decommissioned; both have been defuelled and work is continuing in line with the safestore strategy. Minimising any adverse impacts onthe environment is a key objective throughout this work. Future Technical DevelopmentsOnly 0.7% of natural
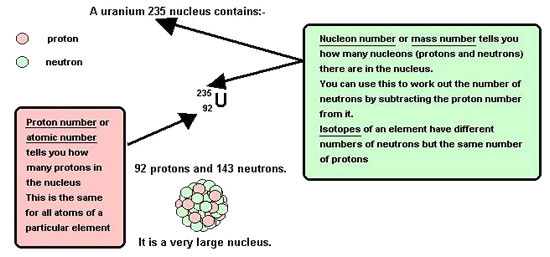
uranium consists of the fissile isotope U235. Existing reactors use enriched
uranium as A kilogram of plutonium-239 can release the explosive energy of 20,000 tons of TNT ,making it the material of choice for fission weaponry. Plutonium was animportant ingredient in the development and production of the first atomicbombs. Plutonium is a highlytoxic radioactive silvery element of the actinoid series ofmetals.It is a transuranium element that has six known allotropic forms.The alpha-version is the one that exists at normal environmental temperatures. It is silvery colour that takes on a yellowish hue as it oxidises in the air. An advanced type of reactor, the Fast Breeder, is fuelled by plutonium extracted from the spent fuel of existing reactors. As well as producing electricity, a fast breeder can also convert depleted uranium, which cannot be used in conventionalreactors, into further plutonium. It can thus produce more plutonium than it consumes, providing additional fuel. In this way, a fast breeder can potentially extract about 60 times as muchenergy from each tonne of uranium as present reactors. If all the depleteduranium in storage in the UK were to be used in fast breeder reactors,it could potentially provide as much electricity as burning 20 billiontonnes of oil (about 7 times the UK's coal, oil and gas reserves). A prototypefast breeder at Dounreay inScotland was closed in 1994, but experimentalfast breeders continue operatingin France, Japan and Russia. However,fastbreeder reactors are unlikely to be economic for several decades. Anotherlong termenergy source, which is the subject of research in the USA, Europeand Russia,is fusion power. In this process,heavy hydrogennuclei are fused together to produce helium nuclei, withthe release of largeamounts of energy. This is the main source of thesun's energy output. Commercial fusion reactors are unlikely to be available until the latter half of this century. |
Follow me...
|



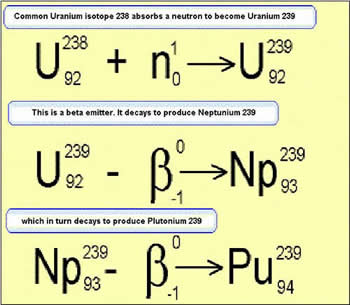 fuel, in which the U235 fraction has been increased to 2-3½%.
During operation of these reactors some of the non-fissile uranium is
converted into plutonium.
fuel, in which the U235 fraction has been increased to 2-3½%.
During operation of these reactors some of the non-fissile uranium is
converted into plutonium.  Plutonium's commercial use is chiefly in
electric power reactors. It is extremely dangerous to handle, and operations
must be done by remote control and with adequate shielding.
Plutonium's commercial use is chiefly in
electric power reactors. It is extremely dangerous to handle, and operations
must be done by remote control and with adequate shielding.


