RMS Speed Questions
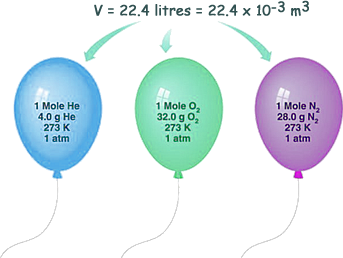 Standard Temperature and Pressure (STP)
Standard Temperature and Pressure (STP)
Q1. Calculate the RMS speed of air molecules in a container in which the pressure is 1.0 x 105 Pa and the density of air is 1.3 kg m-3.
Q2. The following table shows the distribution of the speeds of 20 particles:
| Speed/ms-1 |
10 |
20 |
30 |
40 |
50 |
60 |
| Number of particles |
1 |
3 |
8 |
5 |
2 |
1 |
Find
(a) the most probable speed,
(b) the average speed,
(c) the RMS speed.
Q3. The RMS speed of helium at STP is 1.30 km s-1. Calculate the density of helium at STP.
Q4. The RMS speed of nitrogen molecules at 127oC is 600 ms-1. Calculate the RMS speed at 1127oC.
Q5. If the density of nitrogen at STP is 1.25 kg m-3, calculate the RMS speed of nitrogen at 227 °C.
Q6. Calculate the temperature in degrees Celsius at which the RMS speed of oxygen molecules is twice as great as their RMS speed at 27 °C.



