Charles' Law
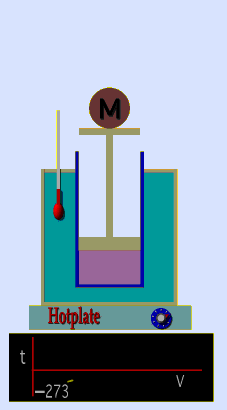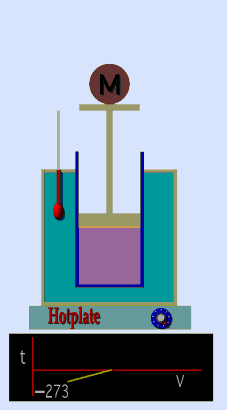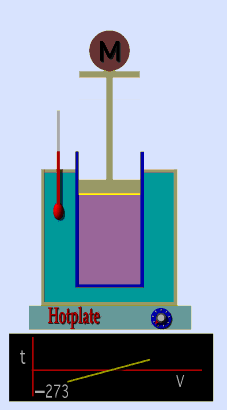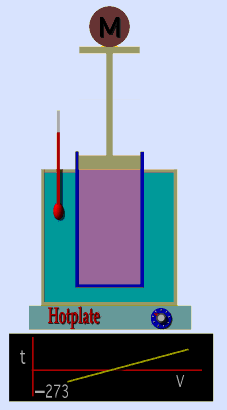
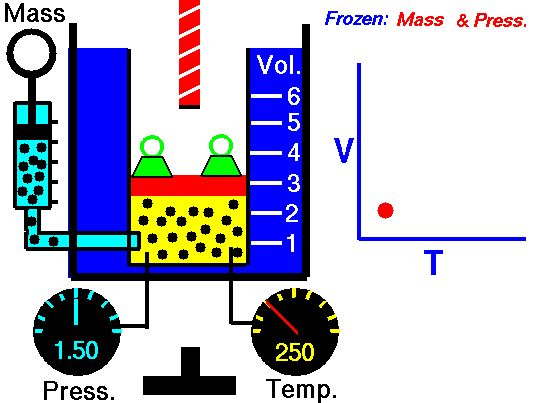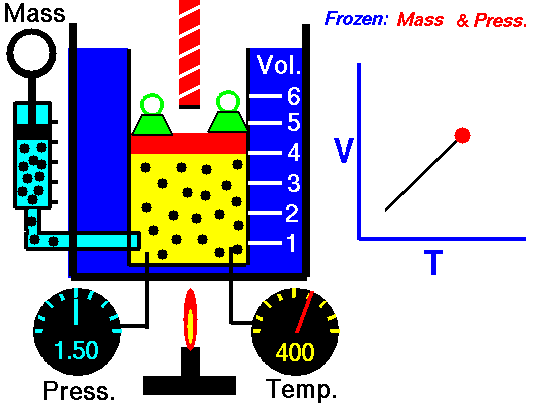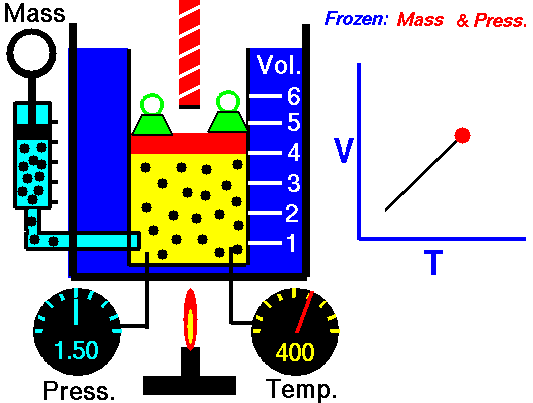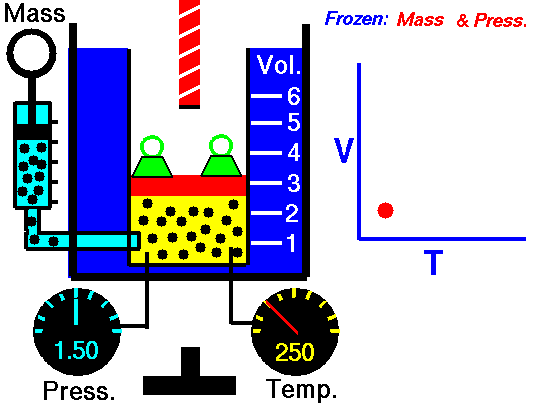
Jaques Alaxandre Cesar Charles (1746-1823) studied the compressibility of gases nearly a century after Boyle put forward his Law. In his experiments he observed that at a fixed pressure, the volume of a gas was proportional to the temperature of the gas. The experiment is simple to carry out. It uses 'atmospheric pressure plus the weight of a mass' as the fixed pressure. A graduated cylinder (one with a scale marked on it so you can read the volume) is fitted with a tightly fitted piston. A fixed mass of gas is trapped under the piston head. The equipment is then immersed in a water bath. A mass is placed on top of the piston which results in a pressure on the gas of the weight of that mass and atmospheric pressure. The gas volume is measured as the temperature of the water bath is increased and volume of gas and temperature is noted down. This is repeated over a large range of temperatures and a graph of volume against temperature is plotted.
If the temperature is plotted in Celsius a straight line graph is obtained with an intercept on the Temperature axis of -273.15 oC. This is the result whatever the mass of the gas and whatever the initial volume. Absolute temperaturesIf we use a scale of temperature that has this -273.15 oC as 0 degrees we can plot a graph that has no intercept. By adding 273.15 to every Celsius reading we can use a new scale - the Kelvin scale - symbol K rather than oC - and we can then say that volume is directly proportional to the temperature - on that absolute scale. Charles LawCharles' Law describes the relationship between the volume and temperature of a fixed mass of gas that is held at a fixed pressure . The pressure is caused by the gas molecules bumping into the walls of the container; so, as the temperature decreases the particles have occupy a smaller volume if the pressure is to remain constant. So the volume occupied by a gas as temperature increases with the temperature of the gas if the pressure is to remain constant. If you increase the one the other must increase by the same factor so that when you divide them you get the same answer. For example, if the temperature is halved, the volume also will be halved. (Note that the 't' in the graphic above should really be 'T' - generally T is used for temperature and t for time) V/T = constantTaken from the NASA site:
|
Follow me...
|