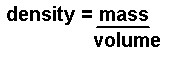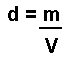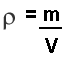Density
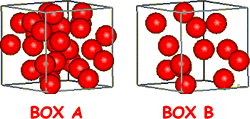
The density of a material helps to distinguish it from other materials. We can list the mass of a cubic metre of lots of materials and compare them. Small items made of the densest materials would still be quite ‘heavy’ whereas large items made of the least dense materials would still be quite light! THE GENERAL EQUATIONDensity tells you how much mass has been packed into a given volume of space.
At KS3 this is abbreviated to:
but at A level we use the symbol 'ro' for density (
UNITSIt is usually expressed in how much mass (in kilogrammes) you can pack into one cubic metre:
Values for densities are listed in tables using this unit, so that people can look them up and use them. In exams and tests you will sometimes find it expressed in how much mass (in grammes) you can pack into one cubic centimetre:
Work in whatever units you are given.... don't change from one to another unless you are told to. However there is a relationship between the two units. A kilogramme is a thousand grammes and a cubic metre is a million cubic centimetres, therefore the density in kg/m3 is always a thousand times bigger than the density expressed in g/cm3 PERFORMING CALCULATIONSAlways lay your calculations out carefully.
The density of an object determines whether it will float or sink. See the Archimedes Principle. TO FIND THE DENSITY OF AN OBJECT1. Use a set of scales to find its mass (in grammes or kilogrammes) 2. Find its volume.
3. Calculate its density (as shown above). Related topics: |
Follow me...
|