Electrolysis of Water to Produce Hydrogen
 Electrolysis of water - a solution to our fuel needs?
Electrolysis of water - a solution to our fuel needs?
As a fuel, hydrogen has a high calorific value, yielding about 2.8 times as much energy as an equivalent mass of petrol. So instead of a tank holding 70 kg of gasoline motor fuel, a car running on hydrogen combustion could run a similar distance using only 25 kg of hydrogen. It is because of its high energy value that liquid hydrogen is the normal fuel for spacecraft.
Solar power could be used to provide an electric current, and then hydrolysis could be employed to split water molecules into hydrogen fuel and oxygen (a very useful gas in itself!).
When burnt as a fuel the hydrogen then just produces water... non polluting!
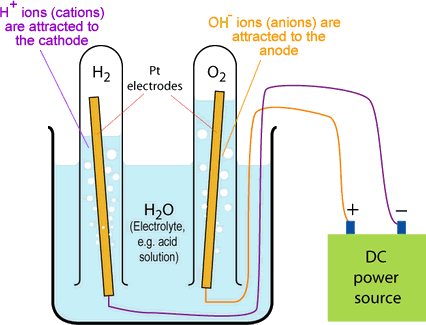
Chemical Reaction in Electrolyte :
Electrolysing water splits the water molecules (H2O) into hydrogen (H2) and oxygen (O2) molecules according to the following equation:
water → hydrogen + oxygen
2 H2O(l) → 2 H2(g) + O2(g)
Reaction at the Anode :
An oxidation reaction occurs releasing oxygen as a gas.
2 OH–(aq) → H2O(l) + O2(g) + 2e–
Reaction at the Cathode :
A reduction reaction occurs releasing hydrogen as a gas and hydroxide ions into the solution.
2 H2O(l) + 2e− → H2(g) + 2 OH−(aq)


