Electron Orbitals
Most popular science pictures of the atom show electrons moving around a nucleus like planets around the sun. These pictures are totally wrong. An old idea about the structure of the atom gave rise to them almost a hundred years ago and they have lasted to this day as a representation of the atom, partly from habit, and partly because the modern understanding of the arrangement of electrons in the atom is too difficult to draw simple pictures of. Orbitals and orbitsWhen a planet moves around the sun, you can plot a definite path for it. That is called its orbit. A simple way in which the atom is explained to you pre-A level makes the atom look very similar to a set of planets orbiting a sun. You may have been encouraged to imagine the electrons orbiting around the nucleus when you were first introduced to the atom. However this picture of the behaviour of electrons is not stricly true. The electrons inhabit regions of space known as orbitals. The word 'orbit' and 'orbital' sound similar, but they have quite different meanings. At a low level allowing you to think of 'orbiting' electrons helps you form a picture of the atom in your mind and can be used to teach you how atoms interact with each other, but at A Level we have to develop a more sophisticated understanding of the topic.
An orbital is the region in space where an electron is most likely to be located. It is not a path, but rather a sketch of the border of the area of space where you have the highest probablity of locating the electron. For a hydrogen atom we have only one electron to consider. If we plotted the location of an electron over a period of time we would get a 3D-plot that looked like a sphere - see the diagram on the right. The spherical shell that outlines the greatest probability of enclosing the electron within itself - say 95% probability of it being within that 'shell' - is called the orbital. It is NOT an orbit or orbital path for an electron, but a probability enclosure for the electron's location.
Exactly what the electron is doing in the orbital, we don't know... in fact we can't know. This is because as well as having properties of a particle, electrons are waves and our understanding of them only allows us a glimpse of their behaviour. That takes us into the realm of quantum physics. Moving between orbitalsWhat we can say for certain is that if an electron is in a particular orbital it will have a particular definable energy and to move between orbitals it will either take in or give out an 'energy packet' or 'quantum of energy'. If it moves to a higher orbital - further away from the nucleus - it will need to absorb a photon of energy to do so. If it 'falls back' to a lower energy is will give out a photon of energy, lowering its energy. These energy levels are shown on an energy level diagram. In physics we use these to represent electron orbital transitions. Electron Orbital Transition Calculations
We speak of the electron as being 'bound' by the nucleus - and being 'unbound' when it breaks free. When n = ∞ the electron is 'unbound' - it is free and no longer in an orbital - the atom has been ionised.
That is really all you need to know about orbitals as a physicist to A level. If you are interested in finding out more you can go to this page.
|
Follow me...
|


 Most images of the atom on the internet are wrong!
Most images of the atom on the internet are wrong! 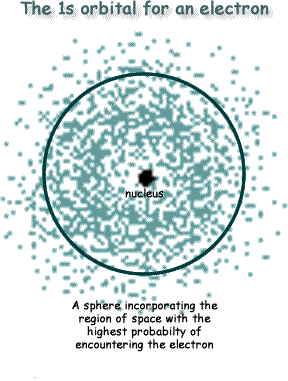 It is impossibile to draw 'orbits' for electrons. To plot a path for something you need to know exactly where the object is and be able to work out exactly where it's going to be an instant later. You can't do this for electrons. The
It is impossibile to draw 'orbits' for electrons. To plot a path for something you need to know exactly where the object is and be able to work out exactly where it's going to be an instant later. You can't do this for electrons. The That is hard to picture (maybe now you understand why your chemistry teacher simplified it as an orbit!)
That is hard to picture (maybe now you understand why your chemistry teacher simplified it as an orbit!)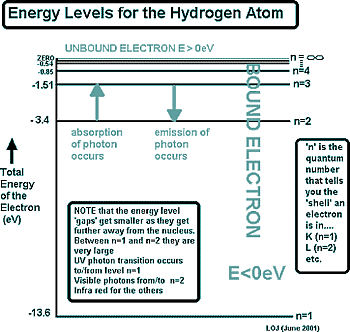 The diagram on the right is for the simplest atom - hydrogen. It is diagrams like this that you will be presented with under examination conditions to calculate the energies of emitted or absorbed photons. From that information you will then have to work out the frequency of the wavelength of the photon.
The diagram on the right is for the simplest atom - hydrogen. It is diagrams like this that you will be presented with under examination conditions to calculate the energies of emitted or absorbed photons. From that information you will then have to work out the frequency of the wavelength of the photon.



