Heating with a Bunsen Burner
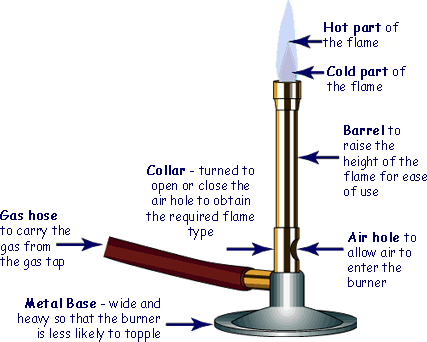
The Bunsen Burner is the most commonly used source of heat in school laboratories.
Natural gas, which is almost pure methane, CH4 is the fuel that you now get from the gas tap in the USA and the UK. Before we 'converted' to natural gas (1970s), coal gas was supplied to the gas taps. That contained hydrogen and carbon monoxide - a poisonous explosive mix!
 Open Air Hole
Open Air Hole
The Bunsen burner has a colar that you can turn to open or close an air hole.
The air hole allows the air from the room to be drawn into the chimney and mix with the gas and therefore allow a plentiful supply of oxygen for the gas to react with. That results in complete combustion.
methane + oxygen → carbon dioxide + water
A very hot, noisy, non-luminous, roaring blue flame is produced. In a brightly lit room this flame can be difficult to see. You should never leave a Bunsen unattended with a non-luminous flame. Someone may burn themselves or set something alight by accident!
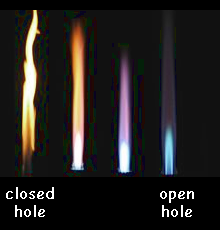 Closed Air Hole
Closed Air Hole
When the air hole is closed the natural gas can only mix with air at the mouth of the chimney. There is therefore not enough oxygen for complete combustion and incomplete combustion occurs.
methane + oxygen → carbon monoxide + carbon + water
A bright luminous yellow flame occurs. This flame is cooler than the roaring blue one and is easily visible. It is sometimes therefore called the safety flame. The yellow flame is produced transfers less heat energy than the blue flame. It is brighter than the blue flame because the specks of carbon soot glow when heated. ut the presence of the soot in the flame means that if you use the yellow flame to heat a beaker of water, you will obtain a very sooty bleaker, tripod and gauze!
Never heat anything using the luminous flame.
Always light the bunsen with this flame. Ensure the colar is closed before you attempt to light it. Collect your flame (on a spill). Turn on the gas, then bring the lighted spill to a position about 2 cm (1 inch) above the chimney.
Half-Closed Hole
 This is best for heating liquids.
This is best for heating liquids.
It does not produce much soot but is not as high energy as the roaring blue flame, therefore the liquid heats more evenly and you get less 'spitting'.
A test tube of liquid should be constantly moved through the flame so that the liquid is evenly heated - small pockets of very hot liquid can expand/evaporate rapidly and spurt up the tube!




 Open Air Hole
Open Air Hole  Closed Air Hole
Closed Air Hole This is best for heating liquids.
This is best for heating liquids. 


