Questions on the Photoelectric Effect
Q2.
The graph shows how the maximum kinetic energy of electrons emitted from the cathode of a photoelectric cell varies with the frequency of the incident radiation.
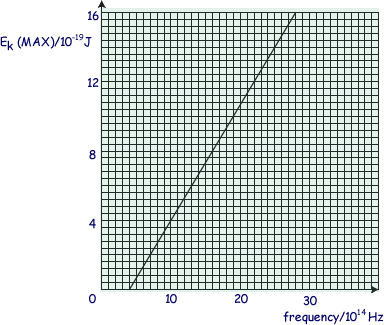
(a) Calculate the maximum wavelength of electromagnetic radiation that can release
photoelectrons from the cathode surface.
f = 4.0 x 1014 Hz. 
c = fλ
λ = c/f = 3.0 x 108/4.0 x 1014 
λ = 7.5 x 10-7 m 
(3 marks)
(b) Another photoelectric cell uses a different metal for the photocathode. This
metal requires twice the minimum energy for electron release compared to the
metal in the first cell.
(i) If drawn on the same axes, how would the graph line obtained for this second
cell compare with the one for the first cell?
The line would be parallel to the original line,  but the intercept on the frequency axis would be at 8.0 × 1014 Hz
but the intercept on the frequency axis would be at 8.0 × 1014 Hz
(ii) Explain your answer with reference to the Einstein photoelectric equation.
The gradient of the line is the Planck constant, h, so it is unchanged  and the intersection with the f-axis is doubled because hf0 = Φ when the photoelectrons have zero kinetic energy - in this case the energy would be twice that of the original if f doubles.
and the intersection with the f-axis is doubled because hf0 = Φ when the photoelectrons have zero kinetic energy - in this case the energy would be twice that of the original if f doubles.
(3 marks total)
(Total 6 marks)


