Wave/particle duality
Q4. The diagram shows some of the electron energy levels of an atom.
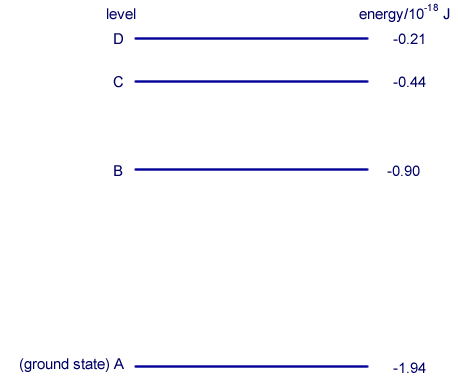
An incident electron of kinetic energy 4.1 × 10–18 J and speed 3.0 × 106 m s–1 collides with the atom represented in the diagram and excites an electron in the atom from level B to level D.
(a) For the incident electron, calculate
(i) the kinetic energy in eV,
Ek. = 4.1 × 10–18/1.6 x 10-19  = 25.6 eV
= 25.6 eV
Ek = 26 eV
(ii) the de Broglie wavelength.
λ dB= h/p = h/mv
λ dB= 6.63 x 10-34/ (9.11 x 10-31 x 3.0 × 106)
λ dB= 2.42 × 10–10 m
λ dB= 2.4 × 10–10 m 
(4 marks)
(b) When the excited electron returns directly from level D to level B it emits a photon. Calculate the wavelength of this photon.
ΔE = (-0.21 - (-0.90)) x 10-18 = 0.69 x 10-18J 
ΔE = hf = hc/λ
λ = hc/ΔE
λ = (6.63 x 10-34 x 3.0 x 108)/0.69 x 10-18 
λ = 2.9 x 10-7 m 
(3 marks)
(Total 7 marks)







