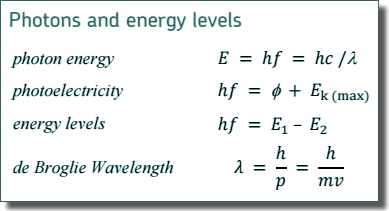
Wave/Particle Dualityde Broglie - Multiple Choice Questions Q1. Electrons and protons in two beams are travelling at the same speed. The beams are diffracted by objects of the same size. Which correctly compares the de Broglie wavelength λe of the electrons with the de Broglie wavelength λp of the protons and the width of the diffraction patterns that are produced by these beams?
The answer is therefore choice A
Q2. A proton moving with a speed 𝑣 has a de Broglie wavelength λ. What is the de Broglie wavelength of an alpha particle moving at the same speed 𝑣?
The answer is choice A.
Q3. Electrons moving in a beam have the same de Broglie wavelength as protons in a separate beam moving at a speed of 2.8 × 104 m s–1 . What is the speed of the electrons?
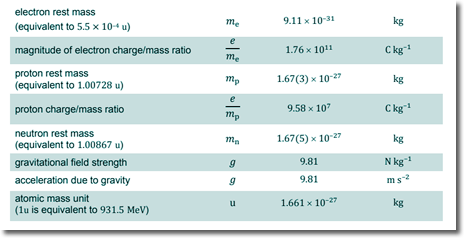
meve = mpvp ve = mpvp/me ve = 1.67 x 10-27 x 2.8 × 104/(9.11 x 10-31) ve = 5.13 x 107 m s−1 Choice D
Q4. Which of the following statements suggests that electrons have wave properties?
|
Follow me...
|