Nuclear Radius
Q6. The high energy electron diffraction apparatus represented in the diagram below can be used to determine nuclear radii. The intensity of the electron beam received by the detector is measured at various diffraction angles, θ.
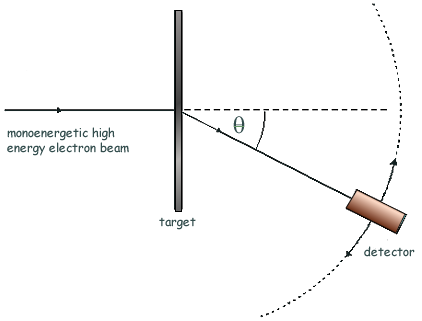
(a) Sketch on the axes below a graph of the results expected from such an electron diffraction experiment.
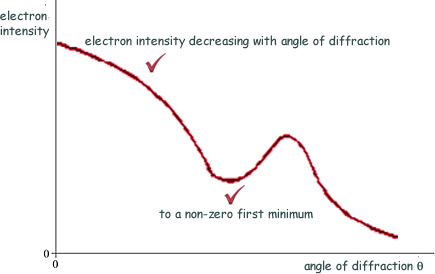
(2 marks)
(b)
(i) Use the data in the table below to plot a straight line graph that confirms the relationship 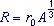
element |
radius of nucleus
R /10–15m |
nucleon number; A |
A1/3 |
lead |
6.66 |
208 |
5.92 |
tin |
5.49 |
120 |
4.93 |
iron |
4.35 |
56 |
3.83 |
silicon |
3.43 |
28 |
3.04 |
carbon |
2.66 |
12 |
2.29 |
element |
radius of nucleus
R /10–15m |
nucleon number; A |
R3/10–45m3 |
lead |
6.66 |
208 |
295 |
tin |
5.49 |
120 |
165 |
iron |
4.35 |
56 |
82.3 |
silicon |
3.43 |
28 |
40.4 |
carbon |
2.66 |
12 |
18.8 |
The first mark is for filling the last column correctly as above - all values! - quoting to the correct number of significant figures and fillimg in the heading correctly.
Two marks are for the plotting of the graph:
 Choice of scale to make good use of the graph paper - axes covering more than 50% of graph sheet
Choice of scale to make good use of the graph paper - axes covering more than 50% of graph sheet 
 Accuracy - all points plotted correctly using correctly labelled axes with units
Accuracy - all points plotted correctly using correctly labelled axes with units
(i.e. x-axis A1/3, y-axis R/10–15m or x axis A, y-axis R3/10–45m3)
(ii) Estimate the value of r0 from the graph.
Stating that the gradient = r0  [or gradient = r03]
[or gradient = r03]
Working out the value from your graph - r0 = (1.1 ± 0.1) × 10–15m 
(5 marks)
(c) Discuss the merits of using high energy electrons to determine nuclear radii rather than using α particles.
 Electrons are not subject to the strong nuclear force so electron scattering patterns are easier to interpret
Electrons are not subject to the strong nuclear force so electron scattering patterns are easier to interpret 
 Electrons give greater resolution
Electrons give greater resolution
or electrons are more accurate because they can get closer
or α particles cannot get so close to the nucleus because of electrostatic repulsion 
 Electrons give less recoil.
Electrons give less recoil. 
 (High energy) electrons are easier to produce than alpha particles
(High energy) electrons are easier to produce than alpha particles
or electrons have a lower mass/ larger Q/m, so are easier to accelerate 
 In Rutherford scattering using α particles, the closest distance of approach, not R is measured
In Rutherford scattering using α particles, the closest distance of approach, not R is measured 
(3 marks maximum)
(Total 10 marks)








