Nuclear Fission
If the overall efficiency of the reactor is 10%, calculate the minimum mass of plutonium required. energy released by the fission of one nucleus of
(Total 7 marks) We first have to determine the time in seconds 100 years = 100 × 365.25 × 24 × 3600 seconds 100 y = 3.155 × 109 s We know that we need power of 300 W - 300 J per second output energy needed if 100% efficient = 3.155 × 109 × 300 It is only 10% effivient so the energy production has to be ten times greater As it is only 10% efficient the energy needed = 3.155 × 109 × 300 × 10 = 9.465 × 1012 J We are told how much energy comes from each fission so we can calculate the number of fissions needed Number of fissions = 9.465 × 1012/3.2 × 10–11 = 2.96 × 1023 The minimum mass would be the mass of that number of plutonium atoms.
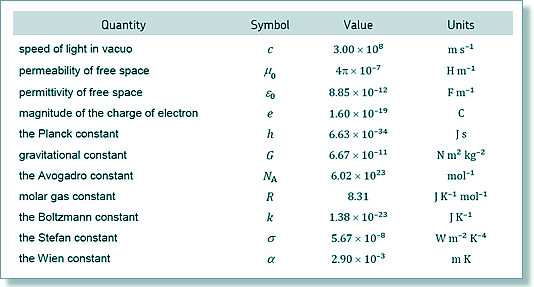
Number of moles = 2.96 × 1023/6.02 x 1023 = 0.492 moles Now 1 mole has a mass of the nucleon number in grammes to the mass of 1 mol Plutonium is 239 g so mass needed is 0.492 x 239 = 118 g (Note that in this case you need to round up - as you are calculating minimum mass that would give that output. If you round down the mass would just short-change the energy required.)
|
Follow me...
|


 Q10. A space probe contains a small fission reactor, fuelled by plutonium, which is designed to produce an average of 300W of useful power for 100 years.
Q10. A space probe contains a small fission reactor, fuelled by plutonium, which is designed to produce an average of 300W of useful power for 100 years.