Nuclear processes - A Level Standard Questions
Q4.
(a) The unstable uranium nucleus 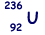 is produced in a nuclear reactor.
is produced in a nuclear reactor.
(i) Copy and complete the equation which shows the formation of 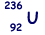
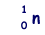 +
+ 

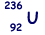

(ii) 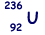 can decay by nuclear fission in many different ways.
can decay by nuclear fission in many different ways.
Complete the equation which shows one possible decay channel.


(2 marks)
(b) Calculate the energy released, in MeV, in the fission reaction.
mass of a neutron = 1.00867u
atomic mass of Ba = 144.92694u
atomic mass of U = 236.04573u
atomic mass of = 86.91340u
Δm = mU – mBa – mKr – 4mn, - the electron masses balance, so can be ignored
Δm = 236.04573 – 144.92694 – 86.91340 – (4 × 1.00867) 
Δm = 0.17071u 
Q = 0.17071 × 931.3MeV = 159 MeV (1)
(3 marks)
(Total 5 marks)


