Atomic Structure
Q9.
(a) A stable atom contains 28 nucleons.
Write down a possible number of protons, neutrons and electrons contained in the atom.
(2 marks)
(b) An unstable isotope of uranium may split into a caesium nucleus, a rubidium nucleus and four neutrons in the following process.
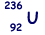

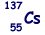

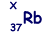

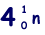
(i) Explain what is meant by isotopes.
(ii) How many neutrons are there in the 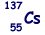 nucleus?
nucleus?
(iii) Calculate the charge to mass ratio, in C kg–1, for the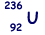 nucleus.
nucleus.
(iv) Determine the value of X for the rubidium nucleus.
(6 marks)
(Total 8 marks)



