Solutions: Atomic Structure
Q3.
(a) The most abundant isotope of cobalt is represented by 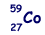 How many protons, neutrons and orbital electrons are there in a neutral atom of this element?
How many protons, neutrons and orbital electrons are there in a neutral atom of this element?
27 (protons) and 27 (electrons) 
32 (neutrons) 
(2 marks)
(b) How is the nuclide that has one less proton than the nickel nuclide,  , represented?
, represented?

(correct nucleon number  correct symbol and proton number
correct symbol and proton number  )
)
(2 marks)
(c)
(i) The heaviest isotope of hydrogen, whose nucleon number is 3, is called tritium. How is tritium represented?


(ii) Calculate the charge per unit mass, in C kg–1, for a tritium nucleus.
charge/unit mass = 1/3 e/mp
= (1.6 x 10-19)/(3 x (1.67 x 10-27)) 
= 3.19 × 107 (C kg–1) 
(allow C.E. from (i))
(3 marks)
(Total 7 marks)


