Kinetic Theory - Multiple Choice Questions
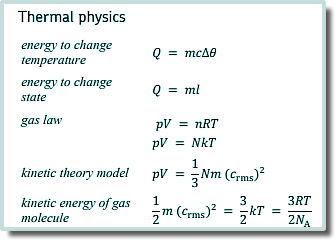
Q1. The height of a water barometer meauring atmospheric pressure at the surface of a lake is 10 m. A diving bell of internal volume 6.0 m3 is lowered into the fresh water lake until the volume of the contained air is 4.0 m3. Assuming the temperature of the air in the bell does not change, find how deep in metres the surface of the water in the bell is below the lake's surface.
Q2. A gas molecule of mass m moving at velocity u collides at right angles with the side of a container and rebounds elastically. Which one of the following statements concerning the motion of the molecule is incorrect?
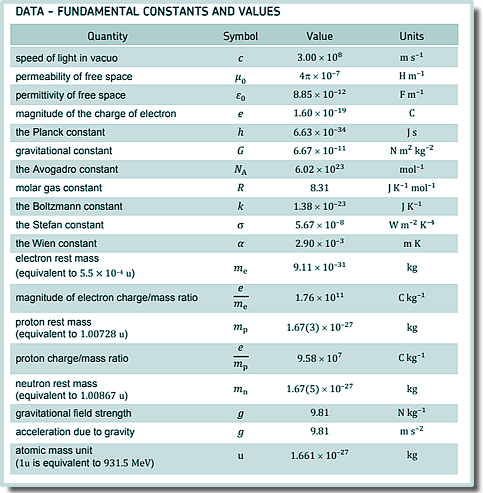
Q3. The composition of a carbon dioxide (CO2) molecule is one atom of What is the number of molecules of CO2 in 2.2 kg of the gas?
Atomic weight = 12 + (2 x 16) = 44 So 44g of CO2 contains NA (the Avagadro number of) molecules.
2.2 kg = 2200 g 2200/44 = 50 ∴ 2.2 kg contain 50NA molecules 50NA = 50 x 6.02 x 1023 = 3.0 x 1025 Choice C
Q4. What is the total internal energy of 2.4 mol of an ideal gas which has a temperature of 15 °C?
Each molecule has an energy of 3/2kT Each mole has NA molecules ∴ 2.4 mol has an energy of 2.4 x 3/2kTNA T = 15oC T = 15 +273K T = 288K E = 2.4 x 1.5 x 1.38 x 10-23 x 288 x 6.02 x 1023 E = 8660 J Choice D |
Follow me...
|