A level: Kinetic Theory Questions
Q4.
(a) The molecular theory model of an ideal gas leads to the derivation of the equation
pV = 1/3 Nm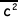
Explain what each symbol in the equation represents.
p is pressure and
V is volume 
N is the number of molecules 
m is mass of one molecule/particle/atom 
 is the mean square speed
is the mean square speed 
(4 marks)
(b) One assumption used in the derivation of the equation stated in part (a) is that molecules are in state of random motion.
(i) Explain what is meant by random motion.
When molecules are in state of random motion they have a range of speeds  and they have no preferred direction of movement
and they have no preferred direction of movement 
(ii) State two more assumptions used in this derivation.
Any two 
 of the following points:
of the following points:
 elastic collisions
elastic collisions
 intermolecular forces are negligible (except during collisions)
intermolecular forces are negligible (except during collisions)
 volume of molecules negligible (compared to volume of container)
volume of molecules negligible (compared to volume of container)
 time of collisions negligible (compared to time between collisions)
time of collisions negligible (compared to time between collisions)
 all molecules identical
all molecules identical
 laws of statistics apply or large number of molecules
laws of statistics apply or large number of molecules
 Newtonian laws apply
Newtonian laws apply
(3 marks)
(c) Describe how the motion of gas molecules can be used to explain the pressure exerted by a gas on the walls of its container.
Molecules are in constant random motion. When the molecules collide (with the walls)  the walls exert a force on the molecules causing a change in their momentum.
the walls exert a force on the molecules causing a change in their momentum.  The molecules exert an (equal and opposite) force (on the walls)
The molecules exert an (equal and opposite) force (on the walls) creating pressure (as pressiure = force/area)
creating pressure (as pressiure = force/area)
(4 marks)
(Total 11 marks)






