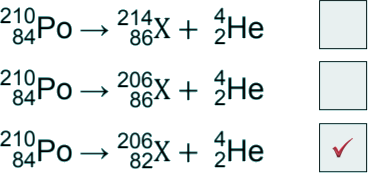GCSE Questions: Radioactivity
Q16. Polonium-210 (  ) is a radioactive isotope that decays by emitting alpha radiation.
) is a radioactive isotope that decays by emitting alpha radiation.
(a) Which is the correct decay equation for polonium-210? (Tick one box).
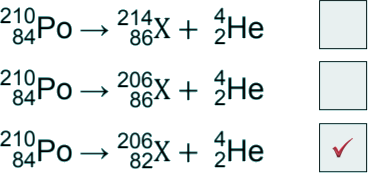
[1 mark]
(b) Why is alpha radiation dangerous inside the human body?
Alpha radiation is highly ionising. 
[1 mark]
(c) The graph below shows how the mass of a sample of polonium-210 changes with time.
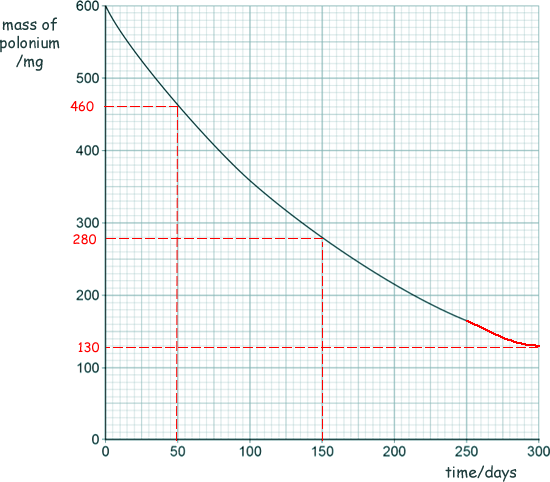
(i) Determine the change in mass of the sample of polonium-210 between 50 and 150 days.
460 - 280  = 180 mg
= 180 mg 
[2 marks]
(ii) Estimate the mass of polonium-210 remaining after 300 days.
130 mg 
[1 mark]
(d) Nuclear radiation can cause ionisation.
(i) How does the charge of an ion differ from the charge of an atom?
An atom has a net zero charge, whereas an ion has either negative (anion) or positive (cation) net charge. 
(ii) How does an atom becomes an ion?
To become a cation it loses an electron.  To become an anion it gains an electron.
To become an anion it gains an electron. 
[3 marks]
(Total 8 marks)