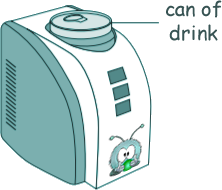
Heat Questions - GCSE Level Q16. A 'can-chiller' is used to make a can of drink colder. The diagram shows a can-chiller.
(a)
[4 marks] (b) Explain how energy is transferred through the metal walls of the can of drink by conduction. In a metal atom there are loosely held 'free' valence electrons [4 marks] (c) Explain howthe energy from the can of drink is transferred to the air around the can-chiller by a convection current that is set up around the can-chiller. As they get warmer air particles become less densely packed
[3 marks] (d) The can-chiller has metal cooling fins that are designed to transfer energy quickly to the surroundings. Give two features that would help the metal cooling fins to transfer energy quickly to the surroundings. A large surface area increases contact and transfer each second. A black/dark dull colouration helps heat transfer by radiation. [2 marks] (13 marks total) |
Follow me...
|