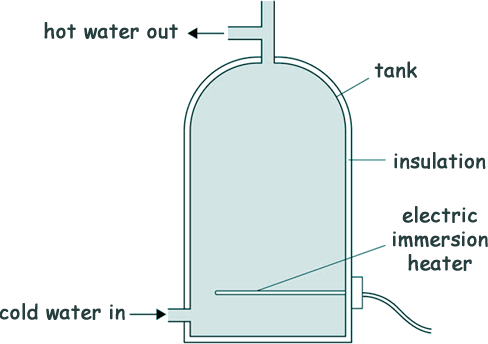
Heat Questions - GCSE Level Q10. The diagram shows a hot water tank made of copper.
(a) Copper has a higher thermal conductivity than most metals. What does that tell us about the rate of energy transfer through copper compared with the rate of energy transfer through most metals?
[1 mark] (b) When the water is hot, the immersion heater switches off. The tank is insulated.
(3 marks) (c)
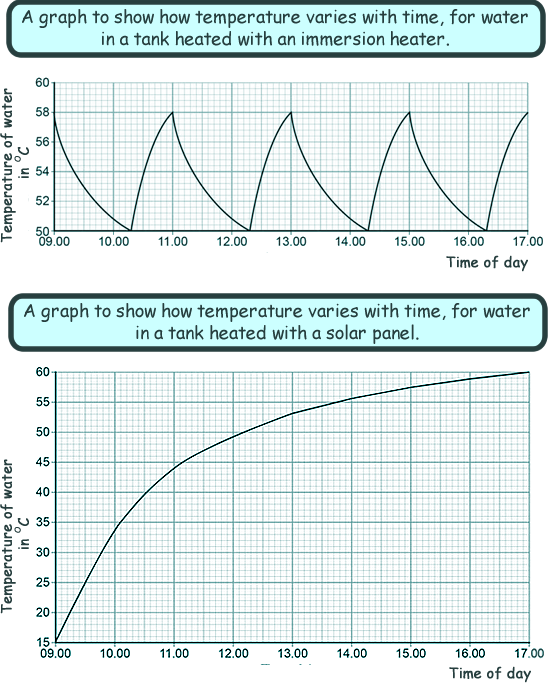
Using only the information from the graphs avove, give one advantage and one disadvantage of heating the water using solar panels rather than an immersion heater. The advantage is that the water heated continuously (by the Sun) throughout the day. The disadvantage is that you have less control over water temperature.
[2 marks] (d) During one morning, a total of 4,070,000 J of energy is transferred from the electric immersion heater. 4,030,000 J of energy are transferred to the water. Calculate the proportion of the total energy transferred to the water. 4030000/4070000 99% of the energy is transferred to the water.
[2 marks] (e) The power output of the immersion heater is 5000 W.
[1 mark]
[3 marks] (12 marks total) |
Follow me...
|