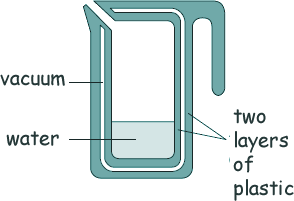
Specific Heat Capacity and Latent Heat Questions - GCSE standard Q7. A new design for a kettle has been suggested. It is made from two layers of plastic separated by a vacuum. After the water in the kettle has boiled, the water stays hot for at least 2 hours. Here is a diagram of the design.
(a) Explain why the vacuum reduces energy transfer to the surroundings and keeps the water warm for a longer time than a traditional kettle. [3 marks] (b) The energy transferred from the water in the kettle to the surroundings in 2 hours is 46 200 J. The mass of water in the kettle is 0.50 kg.
[4 marks]
[3 marks] (Total 10 marks) |
Follow me...
|